A new drug by KalVista is starting a Phase I trial. The drug could become a preventive treatment for people suffering for hereditary angioedema, a rare genetic condition that can be life-threatening.
 Based in the Tetricus Science Park (UK), KalVista targets proteases that are involved in diseases. It develops small molecule inhibitors for plasma kallikrein, an enzyme involved in the dilation and permeability of blood vessels – and may lead to edema and inflammation.
Based in the Tetricus Science Park (UK), KalVista targets proteases that are involved in diseases. It develops small molecule inhibitors for plasma kallikrein, an enzyme involved in the dilation and permeability of blood vessels – and may lead to edema and inflammation.
This strategy caught the attention of investors and last year KalVista managed to raise €30M. Among the investors are Novo (a major investor in innovation in Denmark) and SV Life Sciences, which has around €1.7Bn under management and is behind the Dementia Discovery Fund. With this support, KalVista had already began clinical trials.
Now, it’s the turn of KVD818, a potential for hereditary angioedema therapy (HAE). A Phase I trial has now begun in the UK, and will evaluate the safety and bioactivity (how good it is at inhibiting kallikrein) of the drug. KalVista expects results in the first half of 2017. Should the trial be successful, a Phase II trial is already on the plans.
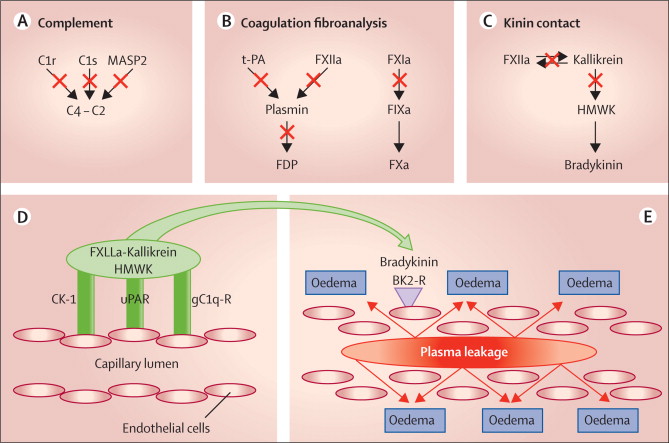
Hereditary angioedema is caused by genetic anomalies that lead to low levels or malfunctioning C1 inhibitor. This affects the permeability of blood vessels. The patients suffer from sudden attacks of edema, which can block their airways or swell the intestines, becoming life-threatening.
KVD818’s sibling for diabetic macular edema, KVD001, has successfully finished its Phase I trial and is moving on to Phase II. Maybe KVD818 will have a similar success…
Feature Image Credit: Red Blood Cells © KTS Design (BigStock ID81484793)
Figure 1 Credit: Longhurst et al. (2012) Hereditary angio-oedema. The Lancet (doi: 10.1016/S0140-6736(11)60935-5)





