Hookipa is starting its first clinical trial for a vaccine against human cytomegalovirus. This is the leading candidate of its platform for novel viral vectors, which promises to yield better vaccines for infections and cancer.
 Based in Vienna, Hookipa was founded in 2011 and is developing a new class of immunotherapies. Its strategy for novel viral vectors already attracted €27M from top Biotech investors in Europe like Sofinnova and Forbion.
Based in Vienna, Hookipa was founded in 2011 and is developing a new class of immunotherapies. Its strategy for novel viral vectors already attracted €27M from top Biotech investors in Europe like Sofinnova and Forbion.
Just last month, the Biotech also appointed new CEO – Joern Aldag, a Biotech veteran who we recently interviewed about his really successful career. Hookipa is keeping its momentum, now beginning its first clinical trial.
The trial is a Phase I study to assess the safety of the Biotech’s leading candidate, the HB-101 vaccine for the prevention of human cytomegalovirus (hCMV). This virus is one of the most significant infections for immunocompromised patients and during pregnancy. As hCMV is still without a vaccine, it has been ranked at the highest priority by health authorities.
It may be unlikely that Hookipa will be the first to get its hands on a hCMV vaccine, however. There are other Biotechs with more advanced programs. For example, AiCuris (interview here) has developed a candidate that is now in Phase III trials and Novartis has a candidate in Phase II.
However, this first clinical trial is a very important milestone for Hookipa. HB-101 is the first result of its viral vector platform (Vaxwave), which has so far gotten promising results in preclinical development.
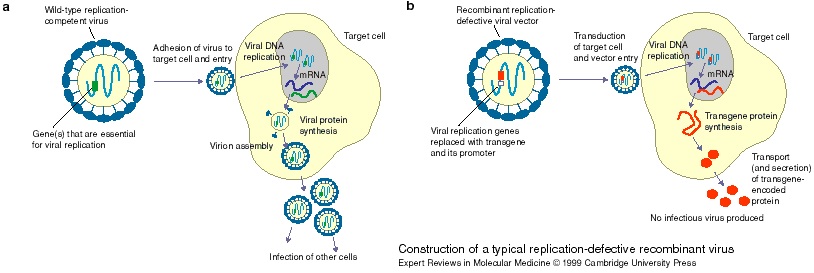
The new class of vectors (engineered lymphocytic choriomeningitis virus) has shown a strong stimulation response of the immune system. Additionally, it doesn’t generate antibodies against the therapy – unlike other vectors. So Hookipa’s vaccines could be administered again and boost protection, instead of being only a ‘one shot’ therapy.
Therefore, HB-101 could have a better profile for protecting people from hCMV. Just as importantly, good results in this clinical program would be a key proof-of-concept for the Vaxwave platform.
Feature Image Credit: Pixabay
Figure 1 Credit: Fry and Wood (1999) Construction of a typical replication-defective recombinant virus. Expert Reviews in Molecular Medicine





