MedImmune and Humabs have reasons to be happy. Their candidate for an Influenza A vaccine has made it into well-known journal, Cell, boasting a unique affinity to the virus with multiple mechanisms to stop infection.
![]() The promising candidate is MEDI8852, an antibody initially developed by Humabs (Switzerland). It targets Influenza A virus (the group of viruses known as avian flu), which is responsible for most influenza hospitalizations and has had health authorities concerned about a possible pandemic.
The promising candidate is MEDI8852, an antibody initially developed by Humabs (Switzerland). It targets Influenza A virus (the group of viruses known as avian flu), which is responsible for most influenza hospitalizations and has had health authorities concerned about a possible pandemic.
MEDI8852 was the result of Humab’s platform to use human memory B-cells from patients that recovered from infectious diseases and isolate their ‘winner antibodies’. Besides Influenza, this strategy also yielded results against cytomegalovirus, Middle East Respiratory Syndrome (MERS) and Ebola.
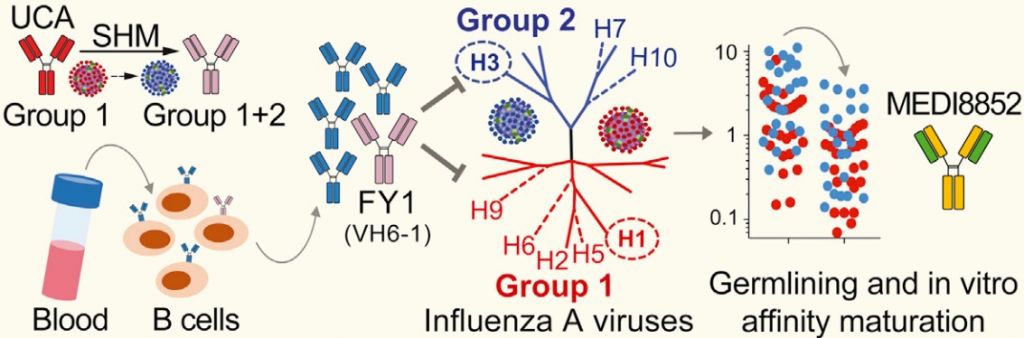
The antibody was later picked up by MedImmune, which is now conducting Phase Ib/IIa trials. The results so far had already earned it a Fast Track designation from the FDA.
Now, its promising preclinical results were published in Cell. The research work highlights the candidate’s potential as a therapy against serious infections with Influenza A.
A big part of the potential is due to MEDI8852’s multiple mechanisms of action. It blocks different steps in the infection cycle, such as inhibiting molecules (hemagglutinins) necessary for the virus to enter the cell and begin infection. Another mechanism is to block the exit of the virus, stopping its spread.
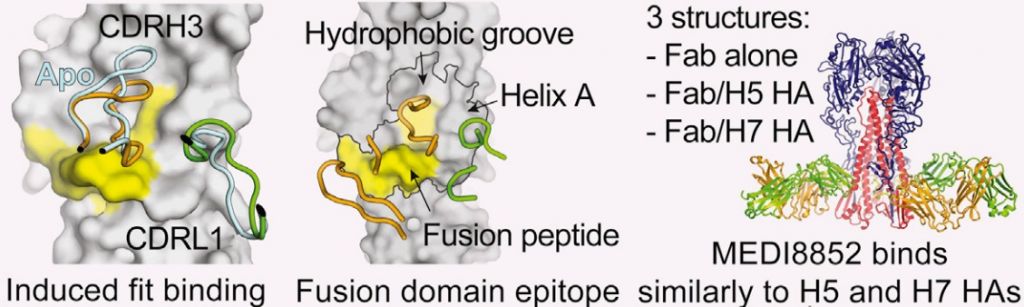
A particular advantage of the targetted epitope is that it is highly conserved epitope across numerous strains, including those with hemagglutinins H5 and H7. This means the antibody should be effective against different versions of the virus and provide a broad protection.
Besides these promising mechanisms of action, the antibody has shown to engage the immune system to eliminate virus-infected cells. Researchers also found it to have a better binding activity than other antibodies, making it more effective. In animal models, it outperformed other therapies that are the current standard of care.
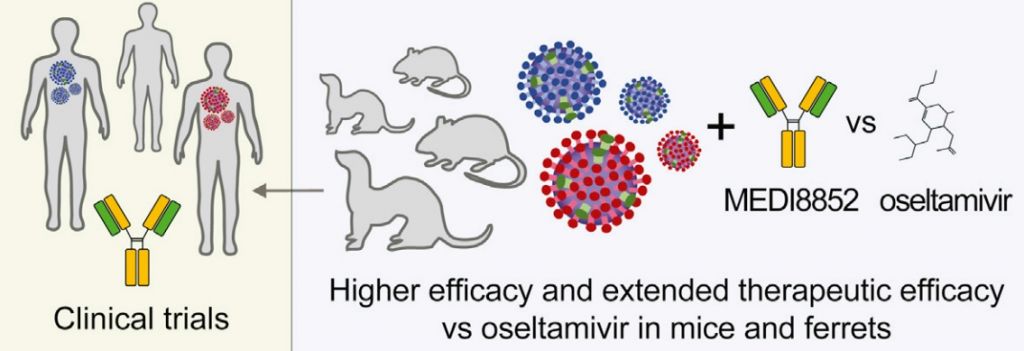
The work was carried out by researchers from MedImmune and Humabs, but also from the Institute for Research in Biomedicine in Lugano (Switzerland) and the Francis Crick Institute (UK).
Along its other successes in clinical development, these results confirm MEDI8852’s promising profile. Given the emergence of drug-resistance, it could fill the high unmet need for a new, universal flu vaccine.
Feature Image Credit: Remix by Labiotech (Background: Pixabay / Medical Vectors: CC 3.0 Servier Medical Art)
Figure 1-3 Credit: Kallewaard et al. (2016) Structure and Function Analysis of an Antibody Recognizing All Influenza A Subtypes. Cell (doi:10.1016/j.cell.2016.05.073)





