While on a stop in New York, I had a chance to chat with Bernard Gilly, the founder and CEO of the Paris biotech GenSight.
 I wanted to learn more about the company, which is one of the only examples in the World using Gene therapy to treat specific and rare types of blindness.
I wanted to learn more about the company, which is one of the only examples in the World using Gene therapy to treat specific and rare types of blindness.
GenSight is interesting in many ways. It was founded very recently (2012) and has since raised a total of €52M in series A & B rounds from some really top-notch VCs: Versant Ventures, Abingworth & Index Ventures (now Medixci).
Bernard Gilly is a veteran of the Biotech space, and even more so in the ophthalmology field. He founded Fovea Pharmaceuticals in 2009, which he sold to Sanofi’s Vision Institute.
He also co-founded the French Pixium Vision (read our take on it here). He has a deep understanding of the field and a extensive experience in entrepreneurship and financing.
GenSight’s technology platform also seems to have immense potential:
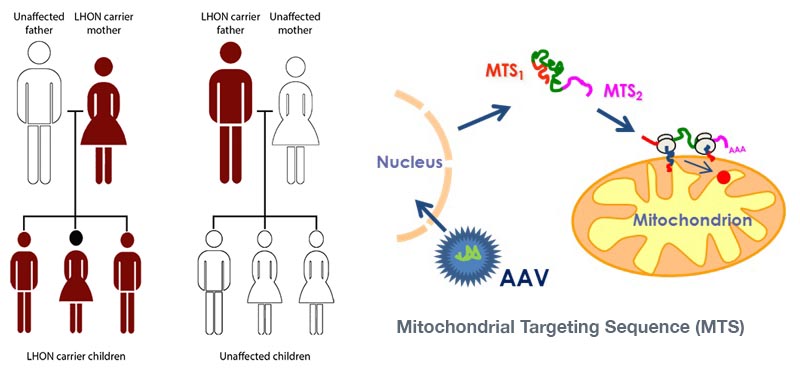
The most advanced program is using the platform to treat Leber’s hereditary optic neuropathy (LHON). LHON affects individuals aged 15-25 and is a brutal, sudden onset disease causing vision loss in both eyes.
It has a staggering 98% probability of complete vision loss within the next 12 months, and roughly 1,400-1,500 people are affected annually in Europe and the US.
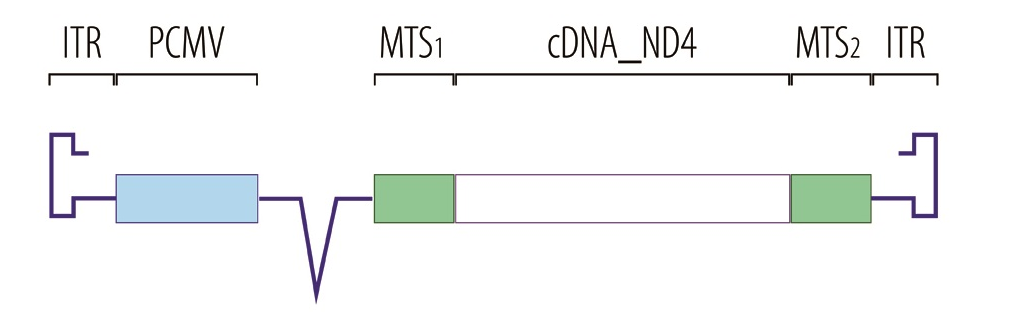
This disease is caused by a mitochondrial dysfunction, blocking the production of ATP. GenSight is therefore targeting the mitochondria with its Mitochondrial targeting sequence technology (MTS).
The therapy is an injectable adenovirus which inserts a specific gene into the nucleus. The resulting mRNA then translocates to the mitochondria and enables the production of ATP again – restoring function to the optical nerve.
So you just announced a new phase III. Can you tell me more about the study and what you expect?
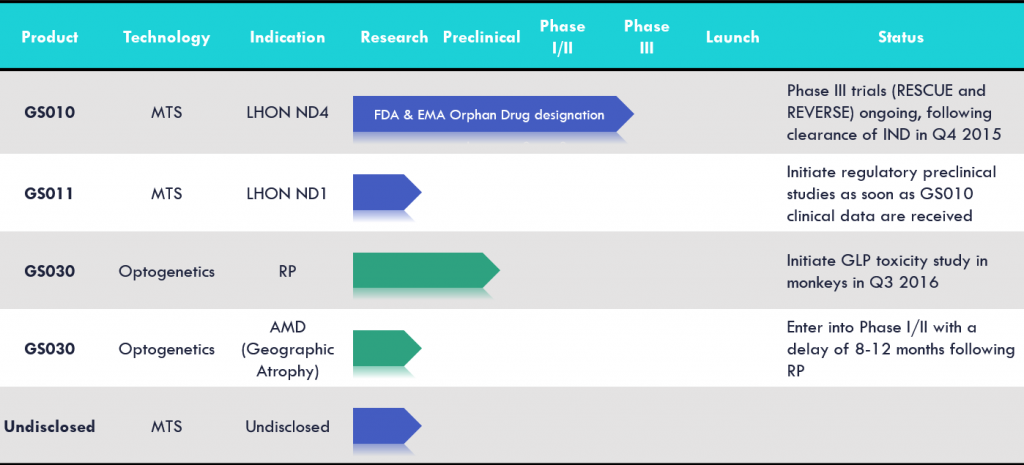
Yes, we just started a parallel phase III trials for our lead product candidate (GS010) to treat LHON, which is an AAV2 vector for the human wild-type ND4 gene. We will target patients in the earlier phase of their disease, 0 to 6 months after the detection, and the patients in the later phase of the disease, 7 to 12 months after detection.
We will have 7 centres worldwide, both in the US and Europe, and expect to recruit 36 patients each trial, for a total of 72 patients. We’ve already injected two patients with the therapy and hope to recruit the next patients soon.
Then, we’ll follow the patients for one year and expect readouts of the study by end of 2017. We hope to have a significant impact on the patients as 50% of the vision is lost within the 6 first months of the disease.
The longevity of gene therapy seems to be one of the main challenge in the field today. How do you overcome it?
Yes, you’re right and that’s why we chose ophthalmology and the central nervous system (CNS). These cells don’t renew or mutate, and therefore should conserve expressions all their life time.
A study on neurons in Monkeys showed up to 8 years of expression of the protein. That’s way longer than other tissues (like the liver), where the best results are reaching 2 years of expression. For example, those of Bluebird Bio in Massachusetts.
The other advantage of ophthalmology for Gene Therapy is that you have access to the tissue to inject the virus. This allows us to get a good rate of spread, leading to up to 30% of transfected retinal cells (cells containing the gene).
We demonstrated this excellent results in a study on monkeys.

I’ve not seen so many Biotech in the ophthalmology space. Who are you competing with?
To my knowledge, we are the only one using Gene Therapy for retinal pigmentosa. A research lab in the US has published some proof of concept as well.
The main focus of Biotech working in Ophthalmology is Age-related macular degeneration (ARMD), where several Biotechs are competing.
But it’s a very complex disease with slow evolution which makes it difficult to tackle. For example, California based biotech Avalanche recently failed to demonstrate real efficacy.
Overall, ophthalmology is a difficult market because it’s mostly made up of rare diseases, and you need a specific knowledge base where there is little overlap with other indication fields.
Last question, I’ve seen you’re also working on Optogenetics (using light to stimulate the brain). While I was in Boston, it seemed to be really booming. What’s your view?
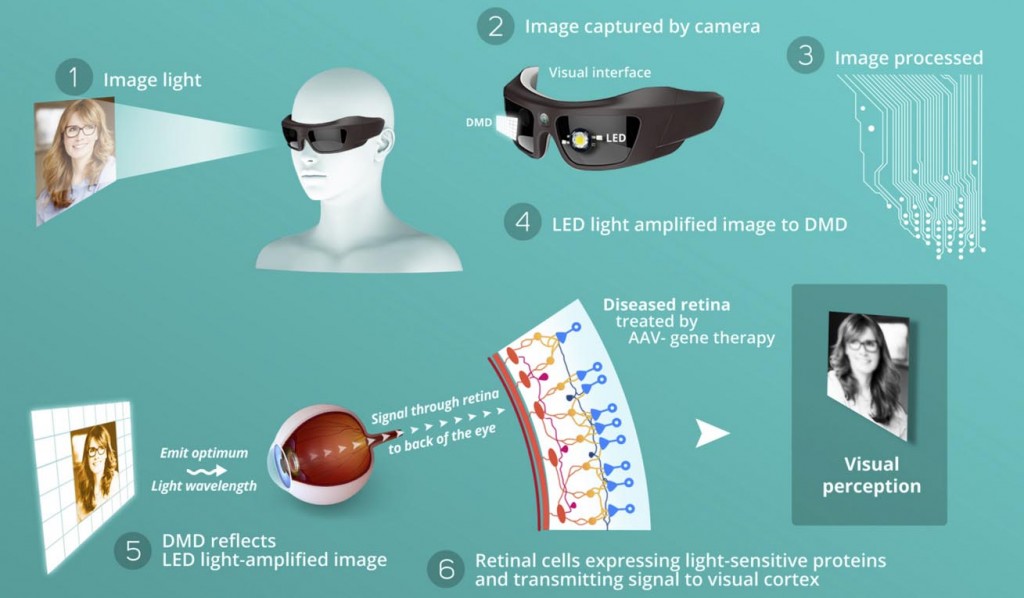
Optogenetics is definitely booming right now as the first proof of concepts are getting published, led by MIT. It seemed obvious to us that optogenetics could have a big impact on ophthalmology as it is exploiting light receptor.
We are using this new technology to fix damage on photoreceptors. We transfer the photoreceptor features into a new cell (thanks to Gene therapy) and inject the cells inside the eye.
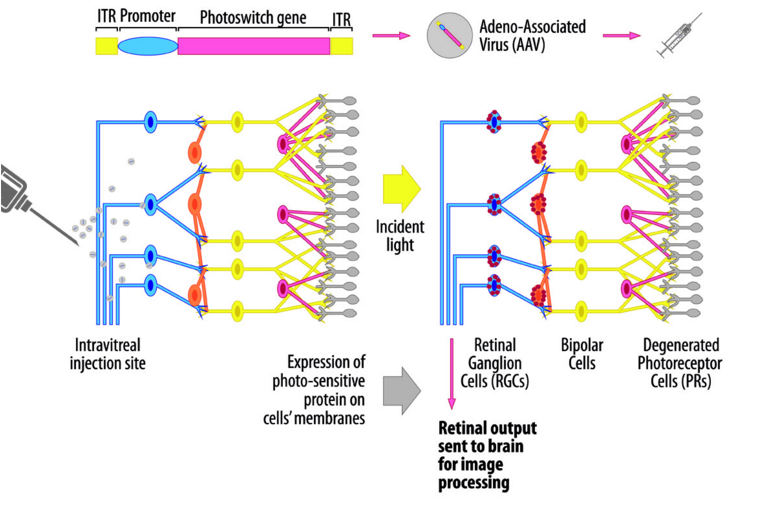
We then developed a pair of glasses (‘the Biomimetic goggles’) that helps redirect and concentrate the light toward the modified cells, which will then convert it into a signal for the brain.
We have first proof of concept in rodent and are now moving to monkeys. We hope to start phase I/II early 2017 and prove that we can give back vision to blind people.
It was great discussing with Bernard, even though the phone didn’t want to collaborate well for the transatlantic communication and it was hard to get everything.
I think GenSight definitely has an interesting platform, which may be combined with the last generation of gene editing technologies to address some of the key diseases in ophthalmology – and on a longer term, the CNS.






