RedHill Biopharma (Israel) is in a solid financial position to reap the benefits of several advanced trials in inflammatory gastrointestinal diseases, including Crohn’s. The company just released its financial statements, with some interesting highlights…
 Based in Tel-Aviv, RedHill is developing small molecule oral drugs for a variety of conditions connected to the gastrointestinal tract. It is listed on the NASDAQ, with a market cap of €120M, but surprisingly just 11 employees (and only 1 in R&D).
Based in Tel-Aviv, RedHill is developing small molecule oral drugs for a variety of conditions connected to the gastrointestinal tract. It is listed on the NASDAQ, with a market cap of €120M, but surprisingly just 11 employees (and only 1 in R&D).
The financial report shows the company is in solid financial state. It has no debts and €53M in cash reserves at the end of 2015, thanks to two public offerings, raising over €50M ($54.7M). It will use this money to further advance its clinical projects.
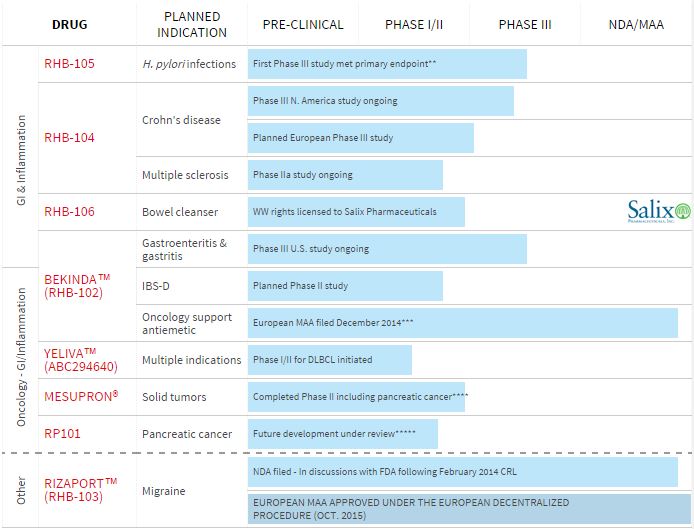
One candidate (RHB-104) is in a phase II trial for Multiple Sclerosis, and another potential indication is Rheumatoid Arthritis. And there are several other advanced projects in the pipeline…
For example, one of RedHill’s focus areas is Crohn’s Disease, for which it is currently holding a phase III trial in the US.
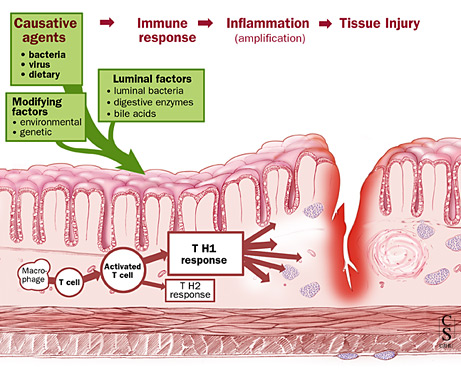
This Biopharma developed a candidate (RHB-104) based on the hypothesis that the disease is caused by a type of mycobacteria, Mycobacterium avium ssp. paratuberculosis (MAP). RHB-104 acts as an antibiotic combination therapy, with antimycobacterial and anti-inflammatory properties.
RedHill has announced that it has completed half of enrolment for the phase III trials, and expects first results in the second semester of 2016.
It is also developing a MAP screening test with US’s Quest Diagnostics, which could ease the diagnostic of Crohn’s – and consequently its own trials.
If successful, RedHill could enter one of the most coveted health markets. Crohn’s disease therapies is one of the fastest-growing therapeutic areas, with a market expected to be worth over €5Bn in 2017 (according to Evaluate Pharma).
With this potential, it’s no wonder a lot of Biotechs and Pharma are investigating Crohn’s Disease. It is one of the primary focus areas of Microbiome leaders like Enterome (France) and Seres Therapeutics (US).
Furthermore, Belgian TiGenix has obtained good phase III results in the treatment of complex perianal fistulas, a consequence of Crohn’s. And filgotinib, the Galapagos‘ (Belgium) candidate that failed in Ulcerative Colitis, has actually obtained positive results in a phase II trial for Crohn’s.
On the Big Pharma side, Roche is currently in phase III trials for its candidate etrolizumab, a monoclonal therapy initially developed by Genentech.
So 2015 was a successful year for RedHill as it was able to raise money before the “financial storm” and finished a phase III trial for H. pylori infections, with good results. Will 2016 be as good? Only the future will tell.





