We met Pierre Belichard, the CEO of Enterome, last week to discuss the increasingly famous trend in the Human Microbiome and how this is impacting therapeutics in Crohn’s disease, the understanding of Medical Microbiomics and investment in this field of Biotech.
Enterome, established in 2012, is a French biotech that is hot at the moment, having already raised €17.5M from a syndicate that included Seventure and two strategic investors, Shire and INRA Transfert. Now Enterome is looking to further develop their Microbiome platform for diseases such as Crohn’s Disease – a severe and debilitating form of Irritable Bowel Disease (IBD).
Pierre Belichard was the founder and COO of Fovea and has been instrumental in building up the product portfolio that became the Ophthalmology division of Sanofi. Pierre has also served as the VP of UroGene, sold to Pierre Fabre in 2004, and Head of European commercial operations at Ethypharm.

So, there are at least 10 Microbiome companies in the World. What do you bring that’s different to the Microbiome Field?
Well, a key area of development includes a discovery platform for new small molecule drugs – which is the defining difference of Enterome from competitors like Seres Health or Vedanta in the US. These biotechs are investigating live bacteria as the ‘next generation’ of treatments for Crohn’s disease.
We, on the other hand, have found that by comparing gut microbe communities between patients and healthy individuals, you can pick up some missing strains and species via a screening process. From these bacteria, we identified several types of regular factors produced by them, including small molecules and checkpoint inhibitors which were missing in patients with certain pathologies.
Our approach allows us to avoid dealing with live culture drugs, which are difficult to develop and deliver to a specific part of the gut. And we do this by attempting to simply replace these missing factors by injecting them directly.
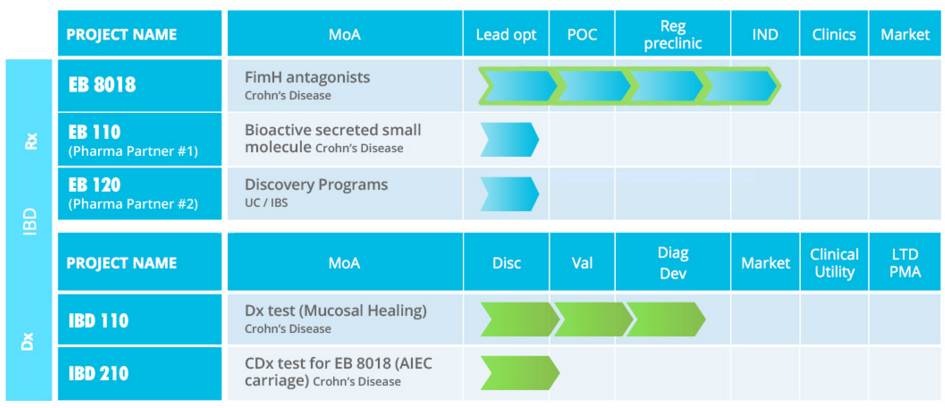
I see Enterome has moved into the Crohn’s Disease Space, why is this such a focal Indication?
The overall Crohn’s Disease market is projected to increase from approximately $3.8 billion in 2011 to $5.6Bn (€5.1Bn) by 2021, so this specialty GI category is growing faster than any other therapeutic area according to Evaluate Pharma (2014).
Generally, what the Microbiome field is investigating regarding Crohn’s is how to treat the disease without killing off all the good bacteria – or result in an imbalance, which can lead to worsening of the gut inflammation.
A course of antibiotics for 1-2 weeks can clear up certain infections caused by bacterial dysbiosis, but if you do this for Crohn’s, there is only a brief period of improvement before the symptoms return and worsen. This is evident in clinical trials.
So what is the Next Step for Enterome?
The goal is to essentially be a fully-fledged pharma company. But the initial step involves settling on a collaboration with a diagnostics partner for our first product. This is a non-invasive biomarker for detection between endoscopy procedures in Crohn’s Disease. If we run this ‘Enterome test’ using shotgun screening of stool samples just the day before a colonoscopy, we can predict the clinical results of up to 84% accuracy.
A collaboration would relieve the burden of building up a commercial study, and we expect to announce the deal in March, although obviously the partner is not yet disclosed and we are still in discussions.
There is also the possibility of a fundraising in a couple of months. We’re also looking to raise in the US and set up an office within the Cambridge Innovation Centre in Massachusetts, where our CBO is based (and I’m already spending about 50% of the time over there too).
So the States appears to be the current future for the company – but we won’t necessarily switch over to the US entirely.
Why Profiling the Gut Microbiome is a Necessity in Microbiome Therapeutics
What about the US competition?
We’re actually very complementary with Seres Health, which are also trying to develop drugs using the Microbiome. Seres’ approach is definitely something which works alongside our R&D – perhaps there is potential for a future collaboration there…
However, on the fundraising level, when we raise €1M, Seres raises more like €10M – but that’s life!”
Nonetheless, we’re still developing and growing very rapidly.
And the rest of the European Ecosystem?
I guess a significant milestone for this ecosystem was the opening of this Microbiome fund (a ‘World First’ which is becoming quite famous). Perhaps this will pave the way for many future collaborations as more companies join in. And this is thanks to Seventure and individuals like Isabelle de Cremoux, attracting initiative in Europe.
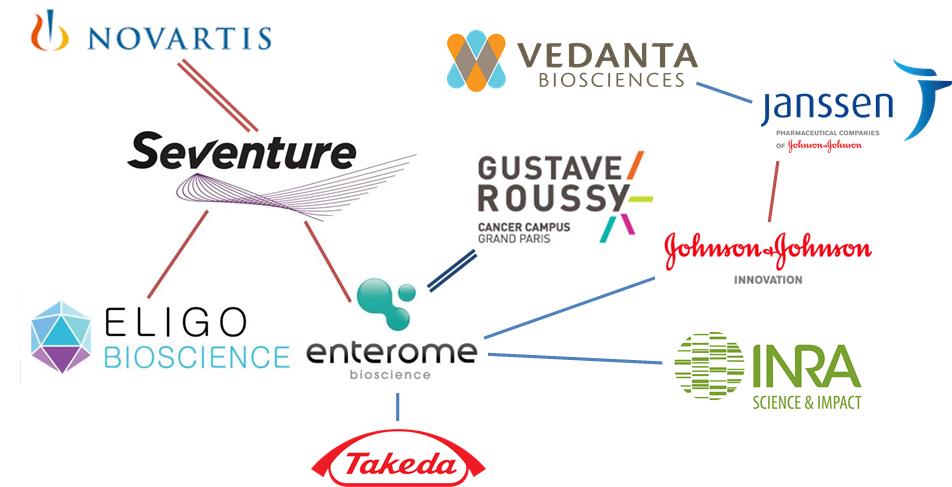
Although I’ve not yet seen so much from Germany yet, there is this new movement in Sweden, with the Karolinska Institutet and then those that are focused on probiotics, such as BioGaia (Stockholm) or Probi (Lund).
So the space to tackle the microbiome this way is still rather open, using a cocktail of small molecules, vaccines and probiotics, and I imagine there will be a number of companies looking to do so in the coming years.
I was really impressed by Pierre’s frank attitude to the competition, and the open vision he holds for the future of such important diseases in medicine. What is even more impressive is that this science and the valuation of the science basis both originate from France, where is an overwhelming example of leadership in the field.
Feature Image Credit: Pierre Belichard (Courtesy of Enterome)





