Change the way we fight against bacterial infections: MICREOS launches its first anti-bacterial enzyme for use in humans against MRSA (Methicillin-resistant Staphylococcus aureus).
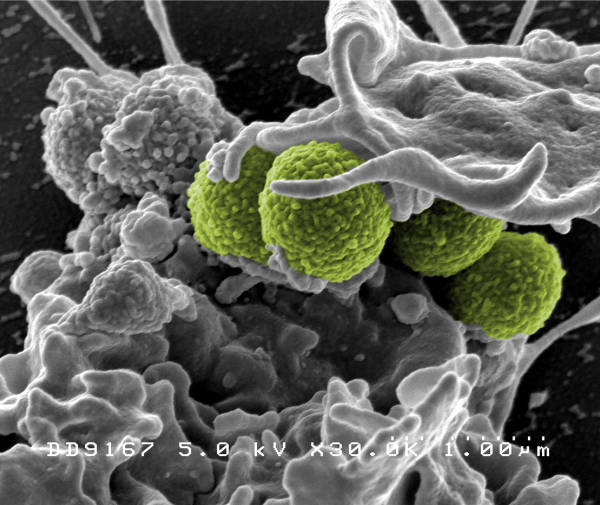
Micreos, a Dutch biotech company, has developed Staphefekt™, a bacteria-killing enzyme specific to Staphylococcus aureus, which is equally effective in killing methicillin-resistant Staphylococcus aureus (MRSA) as methicillin-susceptible Staphylococcus aureus (MSSA).
Emergence of endolysin resistance is not expected and beneficial bacteria are left unharmed by this possible treatment based on the Endolysine Staphefekt ™
Staphefekt™ is the first endolysin available for human use, on intact skin. Endolysins are enzymes that comes from bacteriophages (or phages), microorganisms that kill only bacteria. In nature, phages use bacteria to replicate, in the process destroying the bacterial cell wall with endolysins. The working mechanism of endolysins is unrelated to that of antibiotics, meaning even bacteria resistant to antibiotics are susceptible. Staphefekt™ exhibits several other characteristics: rapid killing (lysis) of the target bacteria and very limited likelihood of emerging resistance, as it works independent of the bacterial metabolism – which harbours the resistance mechanisms – and targets a region of the bacterial cell wall less susceptible to mutation. An additional feature is that its action is specific to S. aureus and does not affect beneficial bacteria.
Dr Bjorn Herpers, Clinical Microbiologist, MD, PhD at Public Health Lab, Kennemerland, speaking at Antibiotics alternatives for the new millennium in London said:
“The results are exciting, and demonstrate the potential this technology has to revolutionize the way we treat certain bacterial infections. With the increasing prevalence of multidrug-resistant bacteria, new strategies for the treatment of bacterial infections are needed. As well as being less prone to resistance induction than antibiotics, endolysins destroy only their target bacterial species, leaving the beneficial bacteria alone.”
In vitro and observational in vivo studies, presented recently in London, confirmed these characteristics. Laboratory results have shown that lysis of S. aureus by Staphefekt™ is specific to S. aureus, efficient, and unlikely to induce resistance.
The number of cases of patients under observation showed similar efficacy. Observational patient case series have demonstrated similar efficacy. In one case series, after the local application of Staphefekt™ for one week, S. aureus was eradicated from the lesions of S. aureus-positive rosacea patients, while other commensal skin inhabitants (such as S. epidermis) remained present. In another case series, S. aureus was found in six skin cultures before treatment (three patients with constitutional eczema, two with contact dermatitis and one with peri-oral dermatitis). In five of six patients, symptoms diminished during treatment with Staphefekt™, and patients reported less or no need for corticosteroids.
Mark Offerhaus, Micreos CEO added: “With the introduction of Staphefekt™, we enter a new era in the fight against antibiotic resistant bacteria, targeting only the unwanted bacteria. This is a far more logical and elegant approach. Millions of people stand to benefit. That’s very exciting and gratifying.”
Current data demonstrate efficacy of Staphefekt™’s bacteria-killing action. Micreos has started clinical trials for new therapeutic areas, and is looking to collaborate with clinicians internationally to establish further trials.
References:
1 – Data Archive Micreos
2 – Herpers Bl et al. Specific lysis of methicillin resistant and susceptible Staphylococcus aureus by the Endolysin Staphefekt SA.100 ™. 24th European Congress of Clinical Microbiology and Infectious Diseases, 2014