Multiple sclerosis is the most common disease of the CNS in young adults. Researchers may have stumbled across a new approach for its treatment.
Researchers at the Medical University of Vienna have identified a target for the treatment of multiple sclerosis (MS). The research, published in the Journal of Autoimmunity, found that histone deacetylases (HDACs) play a major role in an MS animal model. Follow-up studies will be carried out to find out if this research could provide the basis for a new approach to treating MS.
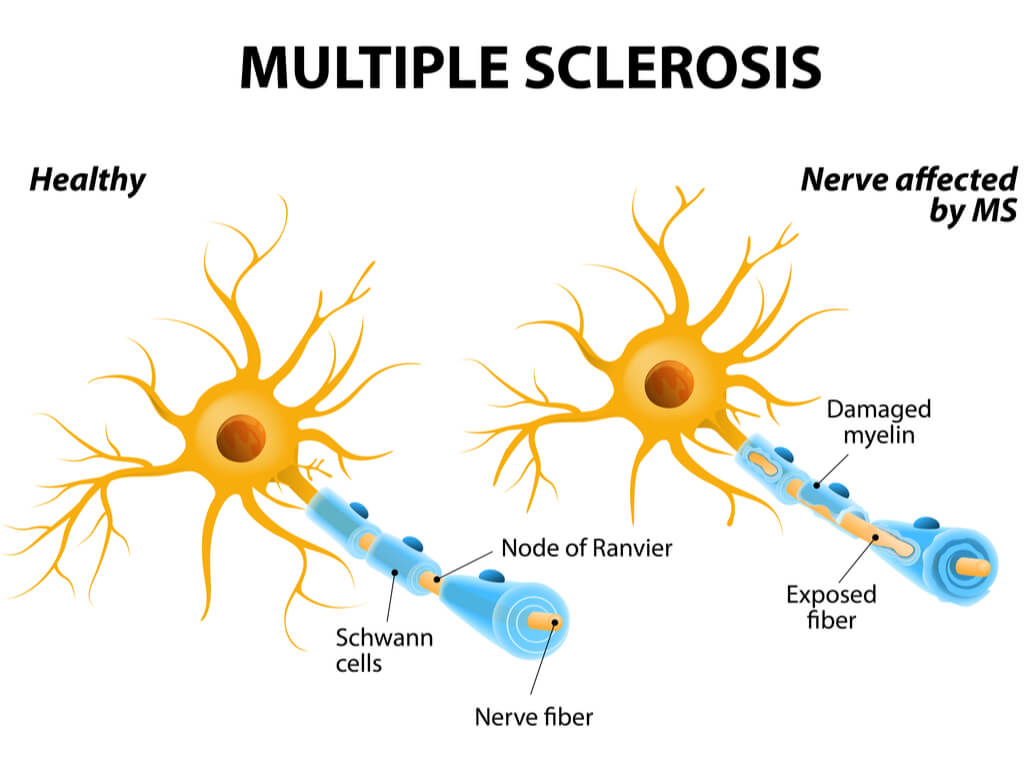
MS affects around 2.5 million people worldwide, which is characterized by an immune-mediated process causing the body’s immune system to act abnormally and attack the CNS. Immune cells attack myelin, a fatty substance insulating nerve fibres. Damage to the myelin sheath or nerve fibre interrupts nerve impulses, giving rise to a range of symptoms, including fatigue, pain and speech and swallowing problems.
The immune system relies on the transfer of information between cells to respond appropriately to dangerous pathogens or cancers. This information takes the form of gene products, and the expression of different genes is regulated by the epigenome – chemical modifications that tell the genome how to behave. HDACs help to tightly package DNA, which prevents proteins from accessing the genome to begin protein synthesis.
Switching off HDAC1 stopped animal models from developing experimental autoimmune encephalomyelitis – an autoimmune disease similar to MS. The group must now clarify how exactly the technique works, Wilfried Ellmeier of the Institute of Immunology, MedUni Vienna, explained: “We still do not know the underlying mechanism responsible for this protective effect… We now want to conduct follow-on studies to discover the molecular details.”
HDACs have been manipulated before for the treatment of cancer, and although often effective, their therapeutic effects are somewhat counterbalanced by serious side effects. This could put other efforts to find an MS treatment higher in the pecking order. Earlier this year, Roche’s candidate, Ocrevus, was approved by the FDA for the based on outstanding Phase III results. Apitope’s peptide vaccine also achieved positive results during a Phase IIa trial.
It is a relief to see biotech trying to find effective treatments to such a devastating disease, and the landmark approval of Ocrevus was a big achievement. The group in Vienna will have to continue testing and refining their research to see if it can be used safely to relieve MS.
Images – RalWel / shutterstock.com; Designua / shutterstock.com





