
BIO-Europe 2015 was a good opportunity for us to meet people who are playing a key role in the Biotech industry. I had the opportunity to meet Miguel Sieler, CEO of Neovacs, a company that can potentially be the first to develop a therapeutic vaccine for lupus patients!
Neovacs is a French biotechnology company developing a new approach: an active immunotherapy. In some autoimmune diseases like Lupus, Crohn’s disease or rheumatoid arthritis, the cytokines that normally activate our immune system are overproduced. This overproduction can lead the immune system to attack healthy tissues and trigger dangerous inflammations. Neovacs’ active immunotherapy uses the patient’s own immune system to regulate inappropriate cytokine overproduction that may occur in autoimmune diseases or even some cancers.
As Miguel Sieler explained to me, “Neovacs’ technology, called Kinoid, is obtained by chemically linking the cytokine of interest to a foreign carrier protein”. When injected into the patient, the B-lymphocytes of the immune system of the patient will develop polyclonal antibodies against the cytokine presented on the carrier protein.
Polyclonal antibodies: a paradigm shift!
This new immunotherapy approach has many advantages. First, the Kinoid technology can be adapted to a wide variety of indications. Once the cytokine responsible for the disease has been identified, the corresponding Kinoid can be developed.
But the greatest advantage is the production of polyclonal antibodies! For several decades, the pharmaceutical industry has focused its innovation on the development of monoclonal antibodies. Monoclonal antibodies have helped a lot of patients in their fight against diseases but these antibodies are not part of the “self” and thus, a resistance against them can be developed by the patient’s body.
The polyclonal antibodies don’t have this drawback has they’re naturally produced by the body. Also, polyclonal means that these antibodies can recognize several epitopes on the antigen at the same time and increase drastically the neutralizing capacity of these antibodies compared to monoclonal antibodies.
Fighting lupus with the Kinoids
Lupus -or more accurately Systemic lupus erythematosus (SLE)- is a systemic autoimmune disease in which the cytokine IFNα is involved. Neovacs has developed the IFNα-Kinoid to fight the lupus. This therapy has already been evaluated in clinical trials and is showing astonishing results!
Last month, Neovacs presented extended follow-up data on IFNα-Kinoid at the 11th International Congress on Lupus. 964 days after treatment, patients enrolled in the Phase I/IIa were still presenting a long-lasting biological activity against all sub-types of IFNα, which is extremely important in this disease. Indeed, Lupus is a disease with flares, meaning sudden and unexpected worsening of the disease. Interestingly, 6 patients are still exhibiting polyclonal anti-IFNα antibodies 4 years after the 1rst immunization and did not experienced flares.
This treatment is of great importance as current therapies for lupus are rare. There is only one monoclonal antibody on the market since 50 years and all recent attempts from pharmaceutical companies did not succeed.
Neovacs is already moving forward to answer this unmet medical need. The company announced on September 24th of this year, the initiation of a phase IIb trial involving 166 patients worldwide.
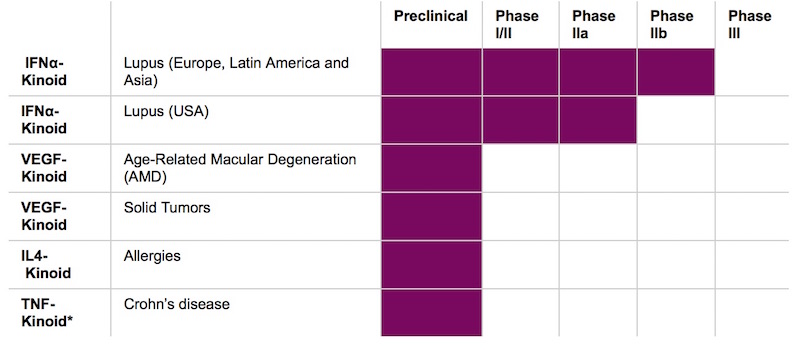
Kinoids, the next generation of immunotherapy
Lupus is not the only preoccupation of Neovacs. The Parisian company is already building a strong portfolio based on the Kinoid technology. Next year, Neovacs will initiate a Phase I/IIa study against Dermatomyositis, an orphan autoimmune disease also recognized as related to IFNα overproduction.
The kinoid technology may also be extended to oncology application in a near future. Indeed, Neovacs is developing a VEGF Kinoïd (VEGF is a cytokine essential for tumor growth).
2015, a new start for Neovacs
In December 2014, Neovacs experienced some severe issues after the failure of its Rheumatoid Arthritis trial. But this failure should not hide the potential of the kinoid-based vaccine approach. The new focus on lupus is definitely the right strategy for Neovacs, as the Phase I/IIa clinical trial suggested as well as the recent results of Anifrolumab (a monoclonal antibody targeting type I IFN receptor) presented this month at American College of Rheumatolgy held in San Francisco by Medimmune.
Last July, Neovacs announced closing of €7.5M capital increase with three U.S. investors specialized in biotechnology. This new funding could even lead Neovacs to be the first company to put a new therapeutic vaccine for lupus on the market.





