Newron sees its Investigational New Drug application approved by the FDA. It can now begin clinical trials for Rett Syndrome patients – a rare neurological disorder.
 Based near Milan (Italy), Newron is a Biopharma developing therapies for diseases of the central nervous system (CNS). Last year it raised over €23M to advance its pipeline, which features candidates for Parkinson’s disease and schizophrenia.
Based near Milan (Italy), Newron is a Biopharma developing therapies for diseases of the central nervous system (CNS). Last year it raised over €23M to advance its pipeline, which features candidates for Parkinson’s disease and schizophrenia.
Now, Newron has received a positive answer from the FDA for its Investigational New Drug (IND) application regarding sarizotan. This therapy is a novel compound for Rett syndrome, which had already been granted an Orphan Drug Designation.
Sarizotan could become the first drug available to patients of Rett syndrome, a rare neurodevelopmental disorder. The disease is associated with mutations in a gene important to neuron function (MeCP2).
Rett syndrome mainly affects females, and is quite rare – one in 10,000. Besides impaired cognitive and motor development, people affected often suffer from respiratory problems, such as hyperventilation and sleep apnea.
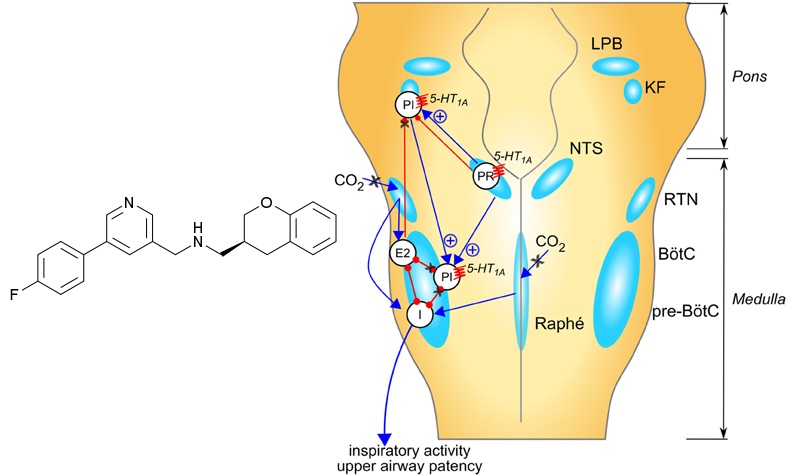
Sarizotan has shown to reduce the number of episodes of apnea in mice models of Rett’s syndrome. With this approval, Newron expects to start a clinical trial (STARS) in the third quarter of 2016.
The STARS trial will be first conducted in the US. Newron said that it is in ‘extensive discussions’ with regulatory authorities.
Newron is taking its newly-found stride to venture in a new clinical development, which could help patients that still have no therapy options available.
Featured Image Credit: Newron
Figure 1 Credit: Cronk et al. (2015) Methyl-CpG Binding Protein 2 Regulates Microglia and Macrophage Gene Expression in Response to Inflammatory Stimuli. Immunity (doi: 10.1016/j.immuni.2015.03.013)
Figure 2 Credit (modified): Abdala et al. (2014) Pinpointing brainstem mechanisms responsible for autonomic dysfunction in Rett syndrome: therapeutic perspectives for 5-HT1A agonists. Frontiers in Physiology (doi: 10.3389/fphys.2014.00205)





