Immunocore in Oxford (UK) has been Granted Orphan Drug Designation for a Phase I Super rare Eye cancer drug by the FDA.
 Uveal melanoma is a rare disease in which cancer cells form in the tissues of the eye, and comprises approximately 3% of all melanomas. It is actually the primary intraocular malignancy of the adult eye and currently, there are no effective treatments on the market for this debilitating disease.
Uveal melanoma is a rare disease in which cancer cells form in the tissues of the eye, and comprises approximately 3% of all melanomas. It is actually the primary intraocular malignancy of the adult eye and currently, there are no effective treatments on the market for this debilitating disease.
Primary treatment has involved enucleation for a long time (such as in the Helsinki Long term study of Uveal Melanoma), so clearly there is a strong demand for an alternative, less scarring form of therapy.
Immunocore is one such biotech we ranked as our Top 10 to watch this Year, so we interviewed their CBO Eva-Lotta last month, and it seems their ImmTAC platform is certainly something to be interested in. Certainly, they were one of the 3 Major Biotechs which ‘overperformed’ last year too, with new partnerships and major progress in pipelines.
Evidently the drug legislation boards agree, with these new qualifications which will hopefully advance their T-cell therapy targeting this form of melanoma which can manifest as blindness.
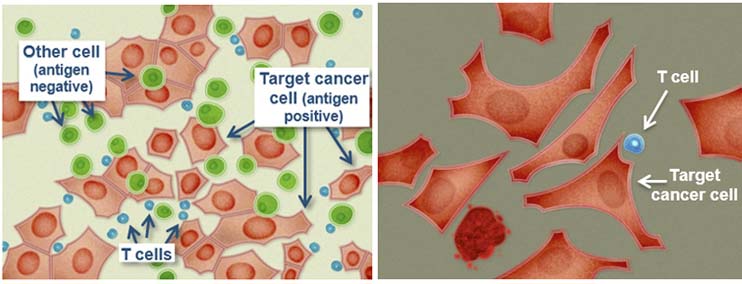
(Source: Immunocore)
IMCgp100 is Immunocore’s wholly-owned and most advanced ImmTAC, currently in Phase IIa clinical trials for the treatment of late stage cutaneous melanoma as well. To date, more than 85 patients have been treated with IMCgp100, which binds to diseased cells and help circulating T-Cells to recognise and attack cancerous cells.
Immunocore has already established deals on IMCgp100’s development for other forms of skin melanoma with major UK Biopharma’s like AstraZeneca and the Biotech MedImmune as a combinatorial therapy. However, it is also being explored as a monotherapy.
Hopefully, with this strong developmental backing and now legislative support, Immunocore can accelerate their potential life (and sight) saving therapy for eye cancer to the patients door.