Apogenix (Germany) has been granted €3M for a personalized medicine to treat glioblastoma. It will also use this funding to develop a companion diagnostic test using a specific cancer biomarker, which other German biotechs are also attempting to do…
 This Heidelberg based-biotech (which we visited on our Labiotech tour of South Germany) is focused on the development of protein Immuno-Oncology therapeutics to treat malignancies, such as glioblastoma, a type of rare brain cancer.
This Heidelberg based-biotech (which we visited on our Labiotech tour of South Germany) is focused on the development of protein Immuno-Oncology therapeutics to treat malignancies, such as glioblastoma, a type of rare brain cancer.
The CancerMark project was initiated in February 2016 by the German Federal Ministry of Education and Research. With a funding period of three years, the goal of the CancerMark project is to confirm the efficacy of Apogenix’s immuno-oncology candidate (APG101).
In an addition, the clinical trial hopes to validate a companion diagnostic test to analyze the biomarker associated with the CD95 ligand, a cell death mediator implicated in cancer.
This is because it was found that the Glioblastoma patients which expressed a certain biomarker associated with the CD95 ligand experienced the greatest benefit from treatment with APG101. Read our review on the value of biomarkers in immuno-oncology to learn more.
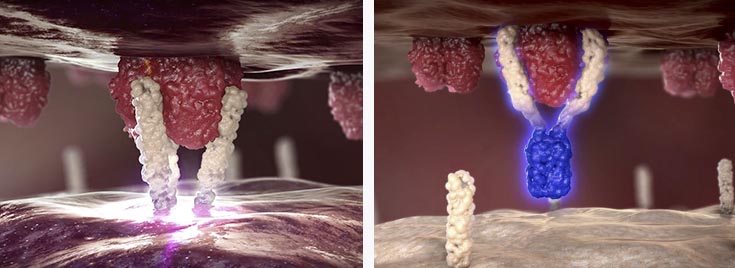
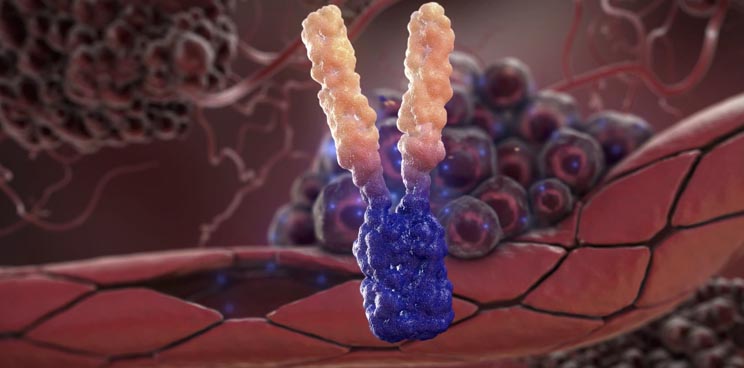
APG101 is a fully human fusion protein that consists of the extracellular domain of the CD95 receptor and an IgG antibody, which is being developed for the treatment of solid tumors and myelodysplastic syndromes (MDS)..
APG101 has demonstrated statistically significant efficacy in a controlled Phase II proof-of-concept trial in recurrent glioblastoma when compared to radiotherapy (with a double overall survival rate in 16 months).
According to Thomas Hoeger, the CEO of Apogenix (whom we interviewed below), the company has now raised more than €11M in public grants for the development of innovative protein therapeutics to treat cancer and other malignant diseases. And this has opened up the way for partnership.
Apogenix’ main partner for the CancerMark project is R-Biopharm in Darmstadt (Germany). In July 2015, the two entered into a cooperation to develop such a companion diagnostic tests for APG101, and around the same time, Apogenix also entered commercialisation partnerships on the Chinese market too.
They are not along though, as two other German biotechs, Immatics (Tuebingen) and BioNtech (Mainz) are working on GAPVAC, a project which is the first EU initiative aimed at biomarker-guided actively personalized vaccines (APVACs) for patients with glioblastoma. GAPVAC received a €6M grant from the European Union.
Evidently, such biomarkers in cancer are a highly desired area of research, given the potential to diagnose these rare cancers earlier and less invasively.