Newsletter Signup - Under Article / In Page
"*" indicates required fields
MiNA Therapeutics is launching the first saRNA human trial of its kind – OUTREACH for patients with severe liver cancer.
 MiNA Therapeutics is a London based RNA specialist, developing RNA activation therapeutics that restore normal function to patients’ cells with an initial focus on severe liver cancer.
MiNA Therapeutics is a London based RNA specialist, developing RNA activation therapeutics that restore normal function to patients’ cells with an initial focus on severe liver cancer.
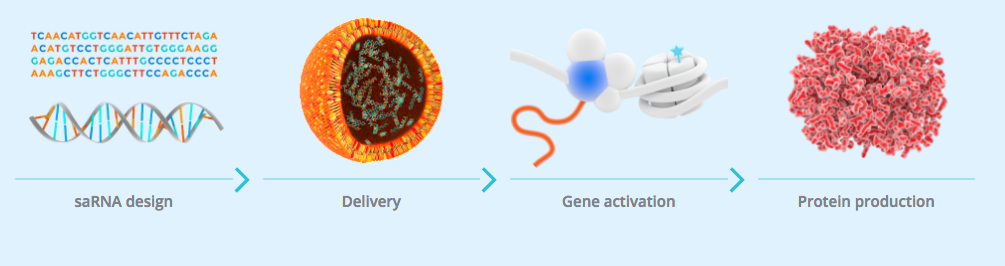
The study is the first-in-human trial of a small activating RNA (saRNA) and is designed to assess the safety and tolerability of their candidate MTL-CEBPA.
MTL-CEBPA consists of a double stranded RNA molecule, formulated into a ‘Smarticles‘ (liposomal nanoparticles) which are designed to activate the CEBPA gene.
The CEBPA gene encodes for the CCAAT/enhancer binding protein alpha (C/EBP-a), a transcription factor that acts as a master regulator of cell lineage determination and differentiation in several tissues including liver, myeloid cells and adipose tissue.
C/EBP-a plays an important role in normal liver function and the benefits of increasing its expression have been demonstrated in multiple pre-clinical models of disease.
The field of mRNA based therapies against cancer is certainly taking off, in part due to the different approach an intracellular mechanism can take in stopping cancer cells. As Ingmar Hoerr, the CEO of CureVac, explained at Labiotech Refresh:
The vaccines are kind of information for the body – showing it how to generate an immune response”.
The multi-centre Phase I study will assess the safety and tolerability of MTL-CEBPA in patients with advanced primary or metastatic liver cancer who are ineligible or resistant to standard therapies.
In the future MiNA Therapeutics hopes to also start trials of this drug for a number of other liver diseases besides cancer, including NASH (non-alcoholic steatohepatitis) and cirrhosis.
Feature Image Credit: Cell-Structure © Eranicle (BigStock ID75460894) adapted with materials source from MiNA Therapeutics (Liposome and DNA + Code)






