Immuno-oncology is definitely the most promising breakthrough in cancer research right now. But the main issue is that only 20-30% of patients respond to this therapy because of a weak immune system. I met Sylvain Carlioz, the co-founder of Stimunity, who wants to solve that problem.
Carlioz was working at the tech transfer office of the prestigious Institut Curie in Paris when a scientist knocked at his door. Nicolas Manel is a group leader at the institute and has patented a new technology to activate the immune system (two papers have been published in Science in collaboration with University of Oxford). “Instead of just helping, I decided to jump on the project and found the startup with Nicolas in April this year,” Carlioz told me.
Immuno-oncology treatments have gone from dream to reality, with immune checkpoints already on the market (CTLA4, PD-1 or PD-L1) or in development (CAR-T and many others), they could be the next generation of blockbusters. Unfortunately, these new therapies need to be improved for patients with weak immune systems (as I explored more in detail here).
“The missing part of today’s immuno-oncology therapies is to make it effective in the 70% to 80% remaining cases“. The goal is to activate a T CD8 answer in order to kill tumor cells.
Carlioz is obviously not alone on that path, since it would lead to one of the most desirable products for Big Pharma. It might also branch out to target cancer vaccines and other immune system activation drugs such as TLR ligands or CpG adjuvant.
Stimunity is going after the STING pathway which according to him “can activate the dendritic cells and the immune system with a low inflammatory signature and open the possibility of higher dose and higher level of activation“.
The startup leverages natural activator of the pathway, cGAMP, that if injected alone, would be degraded and remain extra-cellular (STING is intra-cellular). The company’s innovative solution is to use a virus-like particle (VLP) to encapsulate cGAMP and release it inside the cell.
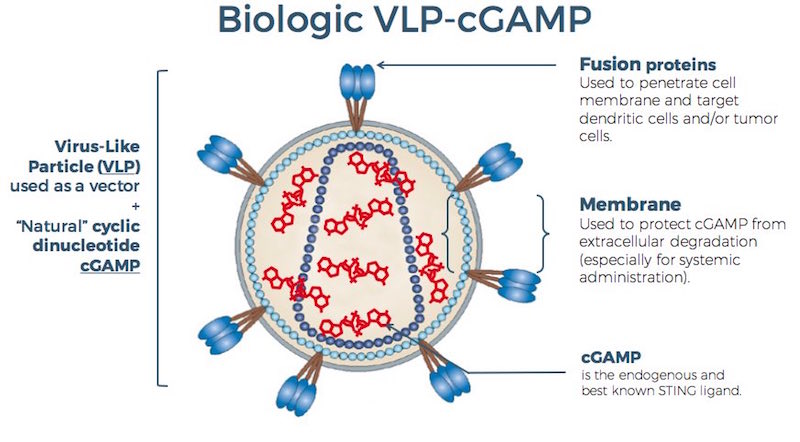
The main competitor of Stimunity is Silicon-Valley based Aduro Biotech, which signed a $750m co-development deal ($200M upfront) with Novartis for a chemically stabilized cGAMP with only pre-clinical data. Novartis justified such an investment with the promise of the STING pathway to help its immune checkpoint and CAR-T programs, the most advanced in the World, even though facing some shuffles last week.
Now, the Parisian startup has a clear differentiation: “Our biological approach dramatically improve efficacy. Our in vitro and in vivo preclinical data showed as same level of activation as existing therapies with 1’000 lower doses.”
The startup is still in the early stage. Carlioz notes, “We’ve just finished the first pre-clinical studies where we were able to show that our approach was effective and is synergistic when combined with checkpoint inhibitors“.
The team is now looking to raise a first €5M round of venture capital to enhance the preclinical data and scale up the manufacturing within the next two years. “We’ve already advanced discussions with early-stage investors in France and hope these additional results will finish to convince them.”
One of the main challenges of the company is the scale-up of the manufacturing and the production of the first GMP batches. These are hurdles faced by most of the new biological treatments (i.e. CAR-T, gene therapy and monoclonal antibodies when they were developed).
The second challenge will be to generate anti-tumor efficacy while minoring side effects in human. Immuno-modulators have already shown fabulous results in animals but it proved poisonous to humans due to high inflammation. “We believe that the high efficiency of our product at low dose is the key to boost the immune system and control the side effects”, says Carlioz.





