It sounds like a lot of fun, but DNA legos are serious: they represent the core concept of synthetic biology, a field with the potential to solve major issues in public health, society and the environment. Here’s a survey of this exciting new area of development.
Major thanks to Eshna Gogia at Helixworks for letting us use her article as a source!
It seems like the stuff of science fiction: lab-grown hamburgers, mushroom leather, bacterial circuits… these can’t be real. Can they? Most of them already are, thanks to synthetic biology, or ‘synbio‘ for short. The field is conceptualised as DNA building blocks or ‘legos‘ that can be built into larger proteins with the goal of building entire lifeforms.
DNA is the foundation of existence for most life forms, dictating an organism’s characteristics and capabilities; genes are then legos that can be assembled to create a distinct entity with completely different functions and systems. Imagine if Dr. Frankenstein built his monster starting from the molecular level! That is the goal of synthetic biology.
Synbio is often aptly described with computer metaphors (the genetic ‘code’ for life, for instance), and the comparison underscores the parallel trajectories of computing and biotechnology towards personalization.
Computers began as tools for exclusive use by military scientists, but gradually made their way to the purview of enthusiasts; half a century later, individual computer use is so widespread that personal machines outnumber people in some countries. The challenge of the 21st century, according to Steve Jobs, is to bring health and medicine to this level of public engagement.
Synbio initiatives are laying the groundwork for ‘open source‘ biology that can be used and customized by individuals to fit their needs, much as has been done with computers. Ellen Jorgenson, the founder of Genspace, eloquently describes this atmosphere and the outlook of synbio in her TED Talk:
How did we get here?
SynBio is a relatively new field, the seeds of which were sown as DNA’s structure and the embedded genetic code were elucidated in the mid-20th century. In the 1970’s, a pair of graduate students named Herbert Boyer and Stanley Cohen showed that genetic material from one species could be inserted into the genome of another: this breakthrough would become the foundation of synbio.

While the raw material existed ten years ago, the field itself did not. Instead, you would have heard about ‘molecular biology’. Only in 2008 did the field of ‘synthetic biology’ appear on people’s lips, when initiatives like IGEM and DIY Bio were launched.
Genetic engineering has today evolved to be more commonly known as synthetic biology. The distinction between molecular biology and synthetic biology is blurred, and in most uses there is no actual distinction. ‘Synthetic biology’ just sounds sexier.” Craig Venter, American Biotech Entrepreneur, in Life at the Speed of Light: From the Double Helix to the Dawn of Digital Life.
This technology has huge potential to combat global issues like food security, energy deficits, water shortages, disease outbreaks and climate change. Moreover, it has changed our approach to the sustainable production of biofuels, food, chemicals and medicine. Let’s take a look at some of the trending synbio projects.
Curing the World’s Ills
Amyris is perhaps the apotheosis of synthetic biology. As one of the fastest growing biotechs, the company scaled up the production of artemisinin, an antimalarial drug that won its discoverers the Nobel Prize in Physiology or Medicine in 2015. Amyris hacked the pathway to artemisinin acid, a precursor to artemisinin, and implanted it in strains of E. coli and modified baker’s yeast to produce it from scratch. Sanofi acquired the program for a cool €150M in 2014 before the company hit a resistance roadblock.
Second Genome is a leading synbio company founded by Craig Venter in 2009 and based in San Francisco. It has been developing a totally new microbiome database to propel the discovery of new therapies and drugs. The company has set out to produce vaccines more quickly and efficiently using their library of synthetic DNA and has raised a total of €52M.
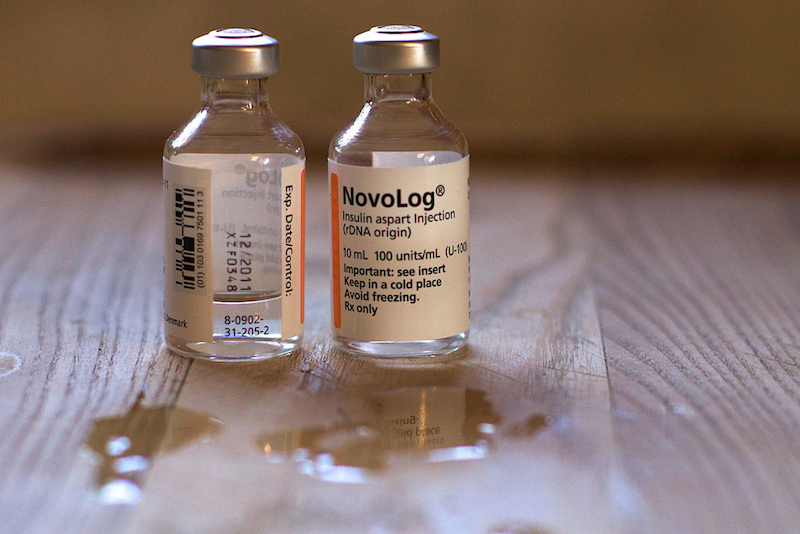
There is no generic or biosimilar insulin available on the market, even though there are over 400M people living with diabetes. Open Insulin is attempting to remedy this need by engineering bacteria to produce precursors of the molecule so that insulin can be easily synthesised and more widely distributed to a broader population.
Eats and Drinks
Meat production negatively impacts the environment and human health, and biohackers have accepted the challenge to ameliorate its effects. Some are producing ‘real’ milk, not soy or almond milk, without a cow or other dairy products. Real Vegan Cheese uses genetically modified yeast to produce casein, a protein flavouring milk and cheese, which they incorporate into their products. Muufri is also taking this approach to make cow-less milk.

Other biohackers are growing meat patties without farming animals for meat: Impossible Foods has identified the molecules behind meat’s taste and add them to plant-based ‘ground beef’. The company’s ‘cultured meat’ has been five years in the making and is far more sustainable, healthy and ethical than its real counterpart.
Sustaining Fashion
MycoWorks is pioneering a leather substitute made from the mycelia of mushrooms and other fungi. These root-like fibres can grow in any sort of agricultural waste and can be moulded into any shape desired. Once it has grown, the mycelium is baked to kill organisms and prevent mushrooms from sprouting.

In a similar but more lucrative vein, Pembient aims to bioengineer substitutes for other luxury wildlife products like ivory and horns. The company was accepted to the IndieBio accelerator early last year.
What’s Next?
A number of organisations like the accelerators and biospaces we mentioned are working on building a bio-interested community for amateurs, entrepreneurs, inventors and really anyone of any age with an interest in biological sciences.
Biotech accelerators like IndieBio and creative biospaces like Genspace are the vanguard inviting nonbiologists to tinker with biology and the technology that builds upon it. For the more dedicated, the International Genetically Engineered Machine (iGEM) competition challenges budding inventors to realize their original ideas and present them in an annual Jamboree.
Just as computer engineering was developed by Jobs to reflect the specific needs of a user, life sciences are increasingly following in its footsteps. The field encourages public engagement and education, local innovation and individual empowerment with possibly huge benefits in not only essential medicine but also crowd-pleasing luxuries like niche foods and fashion to suit every lifestyle and need.
You can hear more about what’s happening in European synbio from our panel of experts. SynBio.info is another great resource to explore the applications of synthetic biology to a variety of fields.
Article Sourced from Eshna Gogia’s article on Medium
Featured Image: Collage by Author – Dna Rendering (CC2.0, ynse/Flickr), DNA (CC2.0, Caroline Davis2010/Flickr), [untitled] (CC2.0, mczonk/Flickr)
Figure 1: Lego DNA (CC2.0, Michael Knowles/Flickr)
Figure 2: Genentech
Figure 3: Life Liquid (CC2.0, Adam Levine/Flickr)
Figure 4: Real Vegan Cheese
Figure 5: MycoWorks






 Genetic engineering has today evolved to be more commonly known as synthetic biology. The distinction between molecular biology and synthetic biology is blurred, and in most uses there is no actual distinction. ‘Synthetic biology’ just sounds sexier.” Craig Venter, American Biotech Entrepreneur, in
Genetic engineering has today evolved to be more commonly known as synthetic biology. The distinction between molecular biology and synthetic biology is blurred, and in most uses there is no actual distinction. ‘Synthetic biology’ just sounds sexier.” Craig Venter, American Biotech Entrepreneur, in