Synlogic, Inc. has announced a new drug candidate for the treatment of gout developed in partnership with Ginkgo Bioworks.
The new candidate, SYNB2081, is a synthetic biotic and is the second product to advance to clinical development through a research collaboration between Synlogic and Ginkgo, following the investigational new drug candidate SYNB1353 for the potential treatment of homocystinuria (HCU).
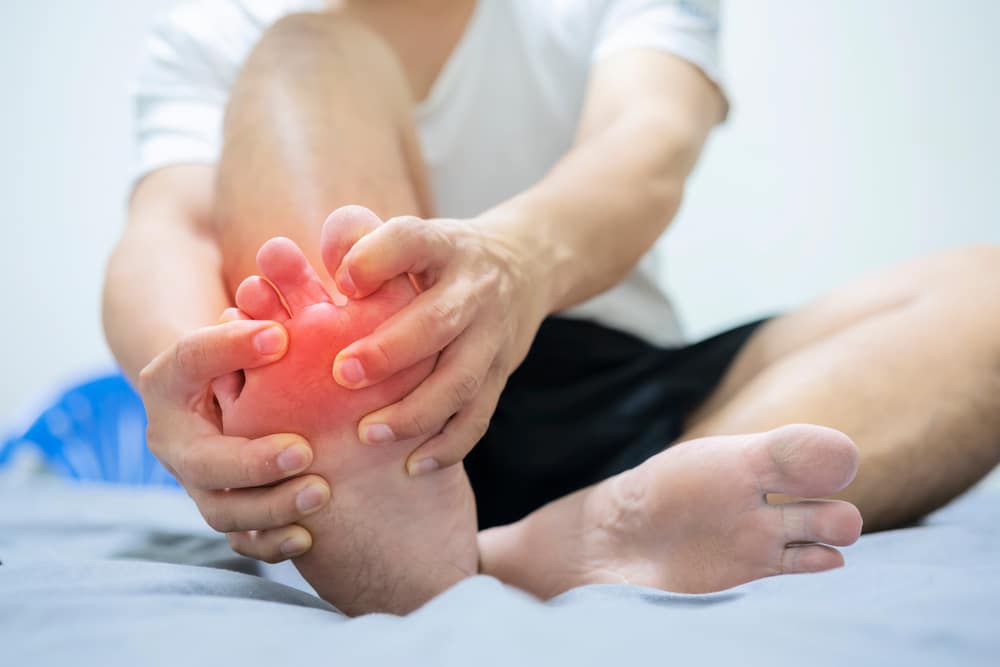
Gout is a complex form of inflammatory arthritis that occurs when excess uric acid in the body forms crystals in the joints. Patients experience symptoms such as intense joint pain, inflammation and redness, and limited range of motion in the affected joints.
Current treatment options present limitations in both safety and efficacy, highlighting a need for new approaches. In addition, gout is a recognized risk factor in chronic kidney disease. SYNB2081 is designed to lower uric acid.
“With our second drug candidate into clinical development, this not only demonstrates the value of combining Ginkgo’s platform with our Synthetic Biotic platform, but also highlights the potential to develop Synthetic Biotics across a range of diseases, giving us the potential to provide meaningful new treatment options to patients in need,” said David Hava, chief scientific officer at Synlogic.
T. rex probably had gout
SYNB2081 is named after one of the largest and best-preserved Tyrannosaurus rex specimens in the world. Nicknamed “Sue,” the specimen is housed at the Field Museum in Chicago and is officially named FMNH PR 2081. Data from “Sue” suggests that dinosaurs like the T. rex suffered from gout much in the same way as other reptiles and birds do.
“The advancement of SYNB2081 and SYNB1353 are clear indicators of the transformative platform Synlogic has created to develop new Synthetic Biotics through synthetic biology,” said Patrick Boyle, head of codebase for Ginkgo.
“We’re honored to work with the Synlogic team in this pioneering next step to potentially help patients living with gout. As we’ve seen the Synlogic pipeline develop over the past year, we’re eager to continue supporting Synlogic in generating additional therapeutic candidates.”