Theravectys just announced positive results of its HIV drug candidate in a phase I/II study. The therapeutic vaccine using lentivirus could be the first cure available for the 35 million people currently suffering from this worldwide epidemic.
Paris-based Theravectys presented detailed results from its phase I/II study at the 2015 Towards an HIV Cure Symposium in Vancouver, Canada. The trial currently enrolled 38 HIV-positive patients under HAART (the antiretroviral therapy currently used to control the disease and not cure patients), and aimed at comparing the safety, tolerability and immunogenicity of the therapeutic vaccine candidate at 3 different doses to placebo.
This phase I/II study is the first-ever lentivector based therapeutic vaccine trial and consists of two intramuscular injections, separated by an 8 weeks time period, of non-replicative and self-inactivating lentiviral vectors. The engineered virus is encoded for immunogenic regions of the HIV GAG, POL and NEF proteins. The treatment aims at inducing a response from the patients own T-cells against these HIV proteins.
With this approach, Theravectys’ goal is to replace HAART with a sustainable cure to the disease.
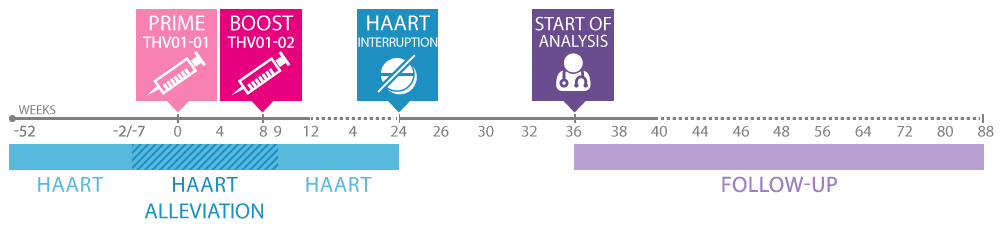
With the absence of any serious adverse events on all 38 patients and no safety concerns related to the treatment, the clinical data confirmed both the safety and tolerance of Theravectys’ lentiviral-based therapeutic vaccine. In addition, the analysis of the immunological data demonstrated the ability of the vaccine candidate to elicit multi-specific and poly-functional CD8 and CD4 T-cell responses in most of the vaccinated patients.
“These data provide further evidence that THERAVECTYS’ regimen vaccine is safe and well tolerated, and can induce intense, broad and long-lasting cellular immune responses in vaccinated patients regardless their preexisting immune profile” says Dr. Cécile Bauche, Chief Scientific Officer of the company.
Theravectys’ ambition isn’t only limited to finding a HIV cure. The company is also developing a robust technology platform with many applications that can be used for infectious diseases or oncology (Theravectys detains for example an Orphan Drug Designation in Leukemia).
As a spin-off of the Pasteur Institute, the company has now capitalized more than 15 years of fundamental research in the field of lentiviral vectors, and has secured worldwide exclusive rights to Pasteur’s proprietary intellectual property related to the use of lentiviral vectors for human and veterinary vaccination and immunotherapy applications.
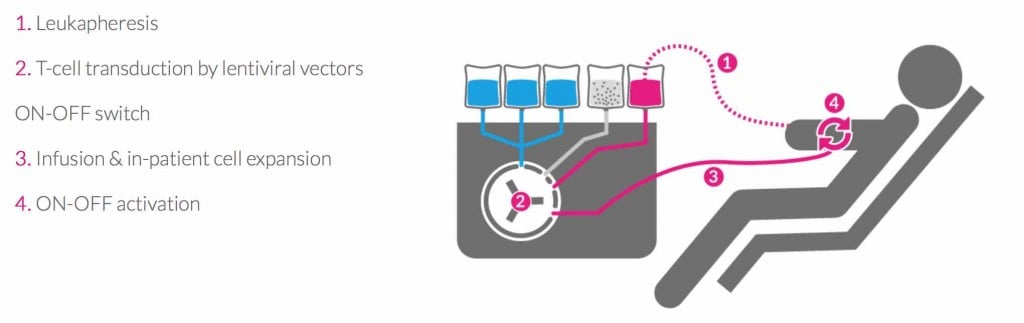
Another reason to keep an eye on Theravectys, is its recent development regarding the trendy CAR-T field. Thanks to its lentivirus expertise, Theravectys can insert a gene into patients’ T-cells and consequently boost them to fight cancers. Even though the therapy is still in a preclinical phase, Theravectys has already imagined an automated at-the-patient-bedside process, which involves an ON-OFF switch that can be triggered by physicians to manage the initiation, intensity and duration of the therapy and thus, avoid CAR-T’s critical side effects such has cytokine storm.
On top of being a pioneer in HIV treatments, Theravectys is building a technology platform that can catch the attention of many Big Pharmas and investors. But with competitive CAR-T approaches using for example the revolutionary engineering tool CRISPR – which doesn’t have the negative reputation of viral vectors – the race to the market will be tough for Theravectys. New and further proof of its technology’s efficacy will have to rapidly follow if the company wants to win this race.