uniQure‘s Glybera was approved in Europe in 2012, making it the first gene therapy on the market in the West. But so far this €1M therapy has only been bought once…
 The approval of Glybera was an important milestone for Biotech. After years of being deemed too risky (after the death of Jesse Gelsinger in 1999), a gene therapy treatment finally made it to the European (and Western) market.
The approval of Glybera was an important milestone for Biotech. After years of being deemed too risky (after the death of Jesse Gelsinger in 1999), a gene therapy treatment finally made it to the European (and Western) market.
Glybera also holds another record. At €1M, it’s the most expensive drug ever. However, according to the MIT Technology Review the drug is a flop. Since its approval, only one patient has received it.
The therapy was developed by Amsterdam-based uniQure, and is a treatment for lipoprotein lipase deficiency (LPLD) – a genetic defect that affects only 1 out of 1 million people. It interferes with fat digestion, and can lead to diabetes and life-threatening acute pancreatitis.
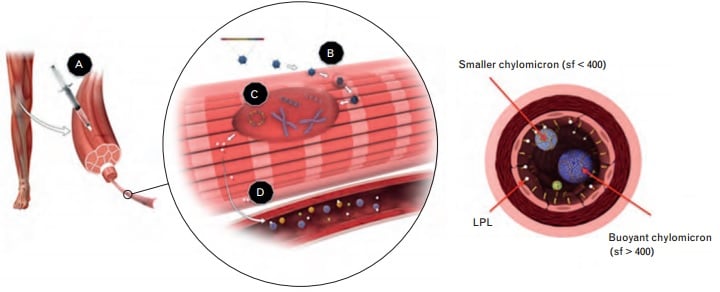
The only doctor (at Charité, in Berlin) that prescribed it said that the process was difficult. She had to personally call the CEO of a top German health insurer to sort out the reimbursement.
While that patient now leads a normal life and the therapy seems successful, the low sales expose a profitability problem for gene therapy.
Glybera cost uniQure over €100M to develop, can only be sold once and has few customers – it is a rare disease, after all. All these factors lead to its price. But that also makes it hard to sell. For exsample, France refused to pay for it.
After uniQure, GSK has also managed to get approval for gene therapy. But it wants to sell it for a lower price, and doesn’t necessarily expect a big profit.
Not all have the same strategy. Spark Therapeutics (US) is almost finishing the development of a gene therapy (for a form of blindness) that could cost $500k per eye. Despite it all, investors are still enthusiastic and there are over 600 ongoing trials for gene therapies.
It’s important to note however, that the problem of drug costs in Biotech isn’t as simple a problem as many think (i.e. this is not necessarily a Martin Shrekli scenario). The process of drug development takes years and millions of euros before any real revenue can be generated (after market approval), and this is in part a barrier to cheaper medicines.
And as for uniQure, it’s unlikely that Glybera will ever pay off. But it still has its trailblazing badge, and its profitable collaboration with Bristol-Myers Squibb has maintained its finances in shape.





