TiGenix is licensing the rights for its cell therapy which is under EMA review and is meant to treat a serious complication of Crohn’s disease. This worldwide exclusivity deal (with exception to the US) could be worth up to €380M.
 Back in 2009, Leuven-based TiGenix was the first to launch at cell-based product (ChondroCellect) on the European market. The Biotech currently develops stem cell therapies for different autoimmune and inflammatory diseases.
Back in 2009, Leuven-based TiGenix was the first to launch at cell-based product (ChondroCellect) on the European market. The Biotech currently develops stem cell therapies for different autoimmune and inflammatory diseases.
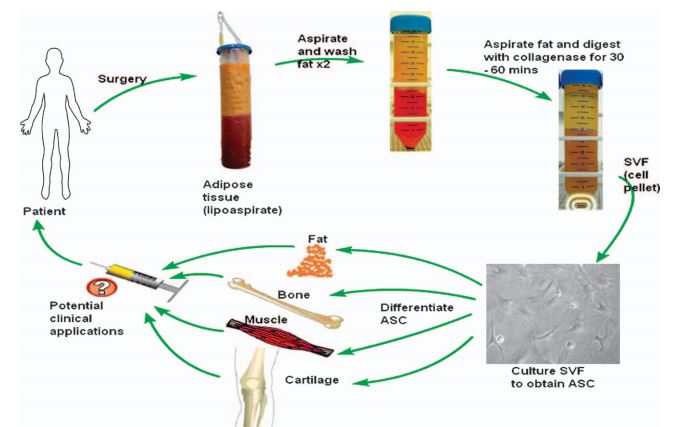
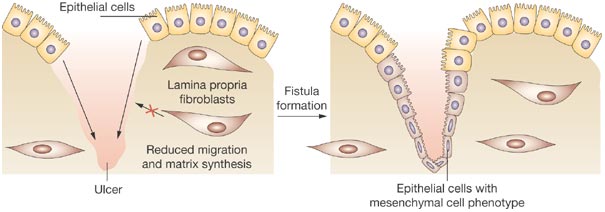
Its most advanced clinical program is Cx601, which has completed Phase III trials almost one year ago, and is now under EMA review. The therapy consists of stem cells derived from the adipose tissue of donors (allogeneic), designed to treat complex perianal fistulas in Crohn’s disease patients, for which there is currently no effective treatment.
Cx601 has now attracted the attention of Takeda, the largest Japanese Pharma, which is paying €25M upfront for the rights to develop and commercialize the therapy everywhere except in the US. Milestone payments could go up to €355M. The first milestone (for €15M) could be just around the corner, as it is the market authorization for the European Economic Area (EEA).
Furthermore, Takeda also expects to invest €10M in TiGenix. It cites its position as “a leader in gastroenterology” as the key motivation for the collaboration. This Big Pharma has shown quite a bit of interest in Biotech for a while now, including its acquisition of cancer-focused Millenium (Boston-based Biotech).
As for TiGenix, this new deal will provide the money necessary to move forward with the clinical development of Cx601 in the US. The country alone represents approximately 50% of the world’s Crohn’s market.
Crohn’s disease is one of the fastest-growing therapeutic areas, with a market expected to be worth over €5Bn in 2017 (according to Evaluate Pharma). So no wonder it’s quite a coveted market, along with other inflammatory conditions.
Other players in Crohn’s disease include RedHill Biopharma (Israel) with an antibiotic strategy, as well as TxCell (France) that is developing T-cell immunotherapies. Additionally, Crohn’s is one of the main targets of the exploding Microbiome field, with impressive collaborations and Biotechs like Enterome.
Feature Image Credit: Pixabay
Figure 1 Credit: Locke et al. (2009) Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ Journal of Surgery (doi: 10.1111/j.1445-2197.2009.04852.x)
Figure 2 Credit: Nielsen et al. (2009) Diagnosis and management of fistulizing Crohn’s disease. Nature Clinical Practice Gastroenterology & Hepatology (doi: 10.1038/ncpgasthep1340)