The Biosimilar drug field is growing fast despite the tight regulatory, business and financial challenges it faces. The industry is forecasted to be worth up to €23Bn by 2020. To explore the market opportunities for biosimilars, it is necessary to be aware of all the latest news and to stand at the forefront of the current landscape of the industry.
BioPharm Insight published a report highlighting some of the major developments occurring in the biosimilar business recently. Some of them were already covered by Labiotech, as the first insulin biosimilar approval in Europe, the first biosimilar approval in the US (here and here) or the world’s first biosimilar monoclonal antibody.
So, first of all, what is it a biosimilar?
Biosimilars are biological drugs that are similar, but not identical to an already-authorized biological treatment (reference product), basically a generic of biological. Because of the difficulty to produce and to characterize these big biomolecules, the security of biosimilars has been questionned.
However, doubts on biosimilars seem to be clearing up as the first ones are getting close to the market, which leads us to the next question:
What is the current situation of the biosimilar market?
The report’s graphs clearly displays the present situation of the field.
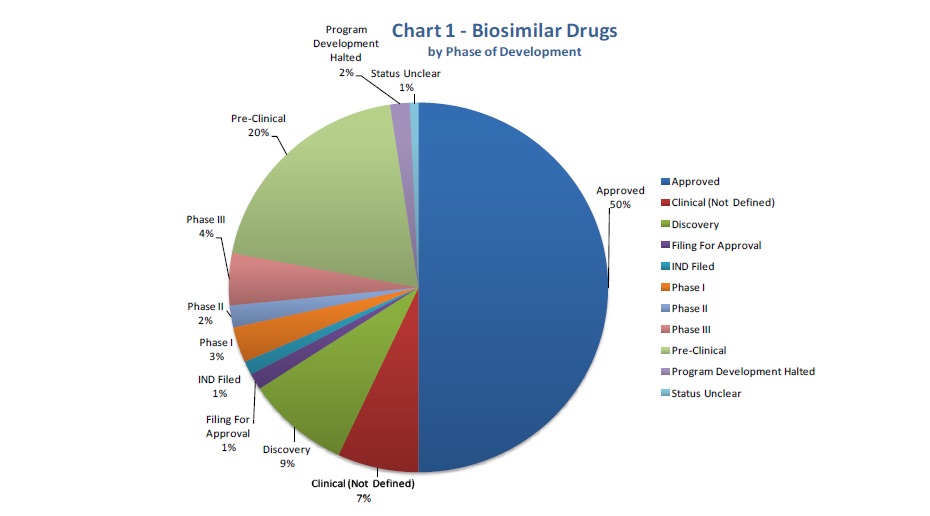
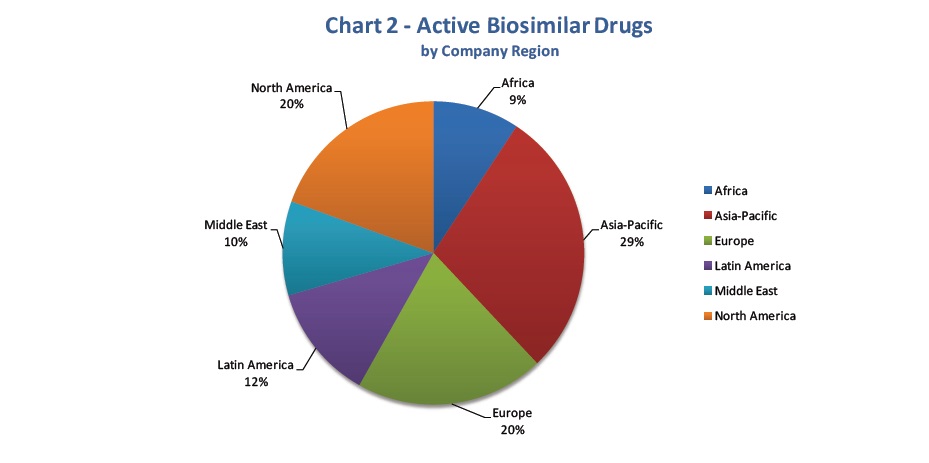
An important 50% of biosimilars has already been approved, correcting all initial fears. On the other hand, the biosimilar drugs’ property is distributed among the different regions of the world, although an Asian-Pacific majority needs to be underlined.
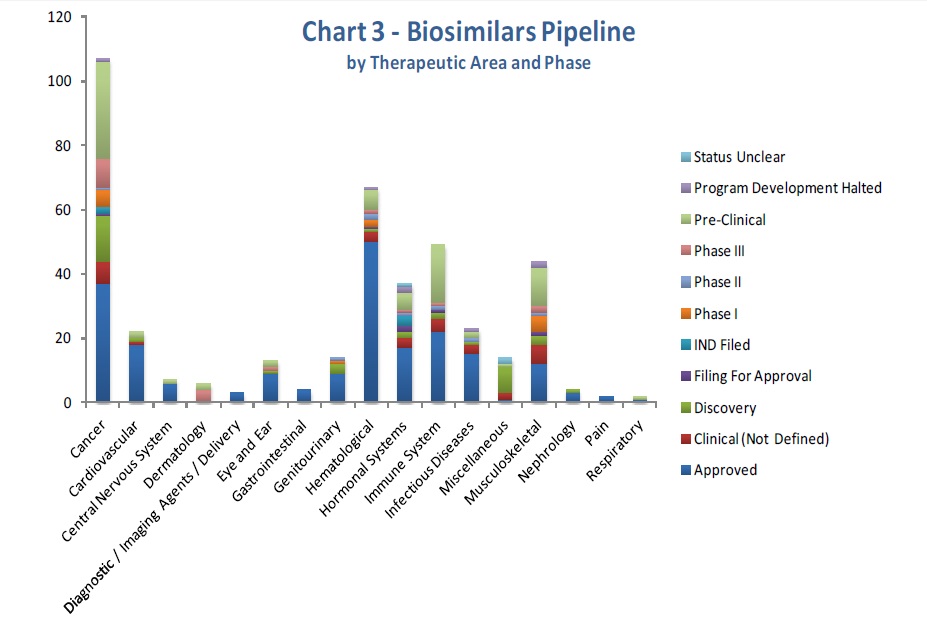
Regarding its application area, the interest of cancer as a target for biosimiliar drugs is clear, followed by hematological diseases. Furthermore, these two areas are also the ones with the highest number of approved biosimilars.
Although the use and uptake of biosimilars has not quite met the initial expectations… they are one of this year’s trends and definitely worth keeping an eye on. I hope this general overview will push your curiosity on this topic further. If so, take a look at the review elaborated by Biopharm Insight for more details.





