Sobi has received market approval by the European Comission for Alprolix, a long-duration protein therapy that protects hemophilia B patients from uncontrolled bleeding.
![]() With a focus on rare diseases, Swedish Orphan Biovitrum (Sobi) has clinical programmes in diseases like alkaptonuria, tyrosinaemia and hemophilia – a disease with which Sobi is particularly invested.
With a focus on rare diseases, Swedish Orphan Biovitrum (Sobi) has clinical programmes in diseases like alkaptonuria, tyrosinaemia and hemophilia – a disease with which Sobi is particularly invested.
Its candidate for hemophilia B (Alprolix) was one of the most advanced. Its long term studies have shown good results, with two phase III trials – B-Long with 123 adult patients and Kids B-Long for children under 12.
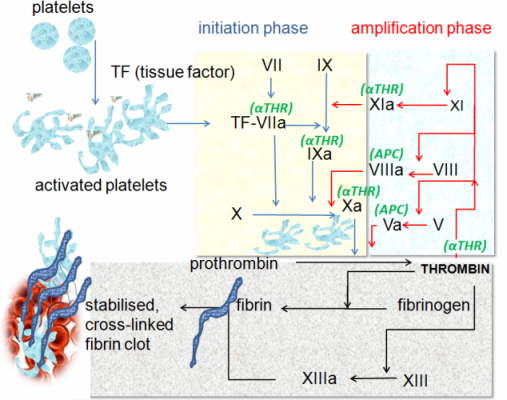
Now, Alprolix (rFIXFc) has been approved by the European Commission (EC). It will be available in all 28 countries of the EU, where it’s the only treatment of its kind for hemophilia B.
The therapy is a recombinant protein that replaces a clotting factor (IX)- which is not naturally expressed in patients. The protein has also been fused to a portion of a common antibody, IgG1.
This modification enables Alprolix to prolong the time the therapy remains in the body – providing longer protection to patients, with fewer injections.
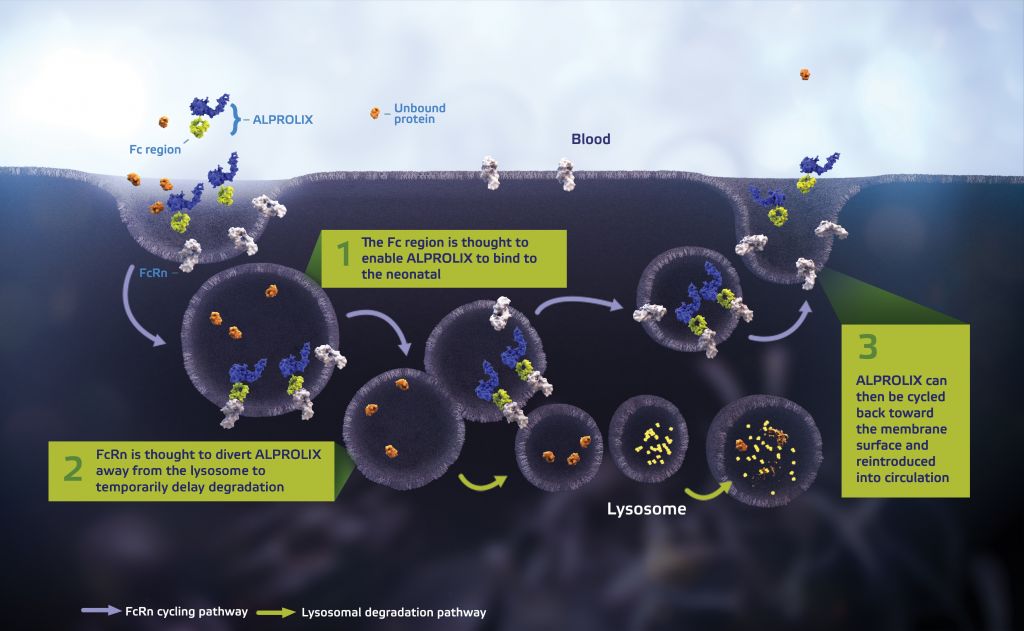
Sobi developed and commercializes Alprolix in collaboration with Biogen (from the US, but expanding in Switzerland). Biogen leads development and manufacturing for Alprolix, while Sobi has rights to final development.
Alprolix had already been approved in the US, Canada and other countries, and is the first advance in hemophilia management in almost 20 years.
Europe is a major market of Sobi’s commercialization territories for Alprolix, so EU approval is a big win for this Swedish Biopharma.
Feature Image Credit: Blood Clot ©digitalista (BigStock ID105438086)