Cellectis is a leader in immuno-oncology CAR-T therapies from France, which might revolutionise cancer treatments. Now, the next step in the CAR-T campaign is to step away from personalised (autologous) therapies, which are time-consuming and expensive, to instead produce an allogenic version available to all.
 So, what is CAR-T? You can read a more in-depth explanation in our ‘Oncology therapies in Biotech’ review here, but in brief – Chimeric Antigen Receptors (CAR) therapies can be engineered to be expressed on specialised T-cells, which can then bind to and attack tumor cells with great specificity.
So, what is CAR-T? You can read a more in-depth explanation in our ‘Oncology therapies in Biotech’ review here, but in brief – Chimeric Antigen Receptors (CAR) therapies can be engineered to be expressed on specialised T-cells, which can then bind to and attack tumor cells with great specificity.
Cellectis had been investigating how to produce Universal CAR-T (UCARTs) antibodies en masse under ‘Good Manufacturing Practice‘ conditions in Industry. Now, their allogenic UCART candidates for blood cancers have been demonstrated to a pre-clinical level, the next step being human trials.
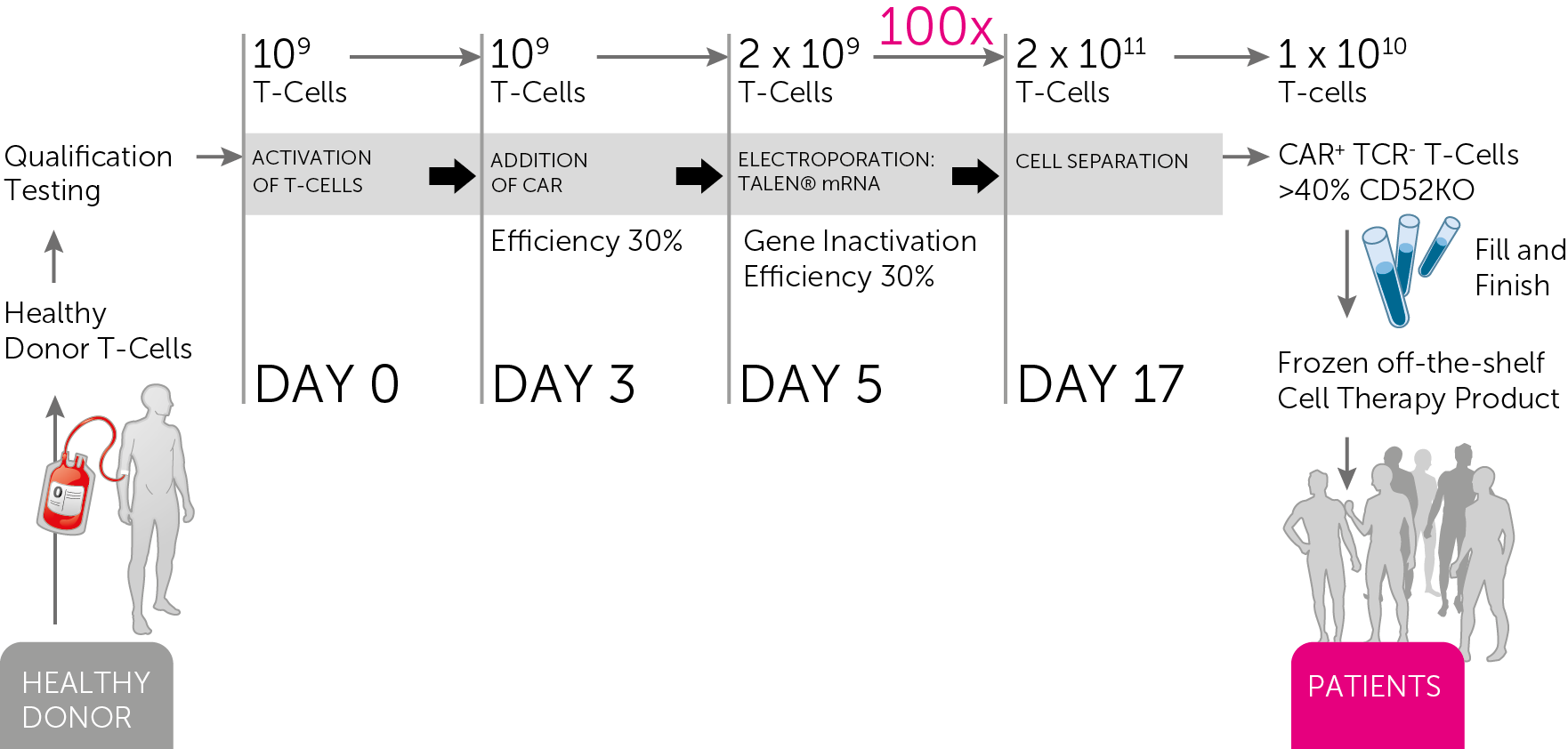
Their focal candidate in question is UCART19, a particular T-cell receptor which has been engineered using their proprietary TALEN gene-editing platform to target Acute Lymphoblastic leukemia (ALL) and Chronic Lymphocytic leukemia (CLL). Their manufacturing process is GMP standard and will increase the efficiency of UCART production so much so that 1 run of the cycle will produce 500-1000 doses of the immunotherapy.
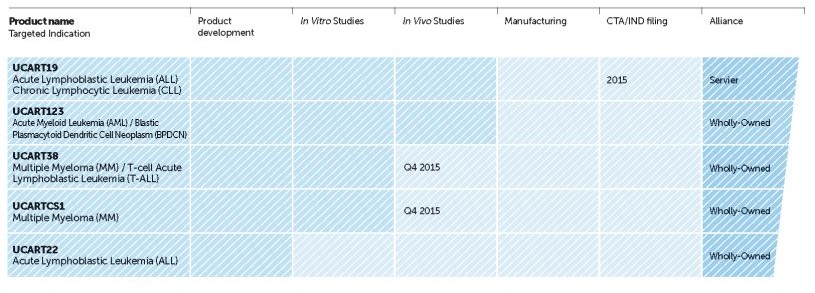
Indeed, this biotech entered a partnership with Servier last February for their UCART work. Despite Cellectis being a biotech worth over €1Bn, the large French pharma Servier actually ended up saving it from bankruptcy. This was mostly due to the discovery of CRISPR in 2011, which has out-competed their gene-editing TALEN platform.
Servier’s €620M investment (including a €8M upfront investment in February last year) has therefore granted them the right to license out UCART19 for commercialization should they wish, and this latest development certainly makes it a more attractive option. Indeed, another pharma giant Pfizer has also been eyeing up Cellectis and their UCART progress.
This latest news is, therefore, a big milestone for Cellectis and their development of a universal CAR-T therapy. Are you prepared for the potential fortune these UCARTs could make?





