What do shuttles, bubbles and ultrasound have in common? They are all methods that can all be used to target the blood–brain barrier and make treating brain diseases such as cancer and Alzheimer’s easier.
The blood–brain barrier is the gatekeeper to the brain. This highly selective protective sheath regulates the flow of substances in and out and prevents toxins and bacteria from entering. Whilst this function is vital for our survival, it presents a massive roadblock in delivering drugs – and treating diseases that affect the brain.
Over the past few years, several broad approaches tackling this issue have been garnering much attention. What are these, how promising are they, and how soon can we expect to see treatments in the clinic?
Peptides as ‘Blood–Brain Barrier Shuttles’
Molecular vectors, known as blood–brain barrier shuttles, are a class of molecules with a drug fused to a small peptide. The peptide is able to bind to specific targets, often transporters, found lining the blood–brain barrier, allowing the drug to be transported. Blood–brain barrier shuttles act similarly to ‘molecular Trojan horses’ – drug candidates attached to antibodies that target the brain — but are typically smaller, less expensive to generate, have lower immunogenicity and are easier to modify chemically. These features hint at the great potential blood–brain barrier shuttles have for drug delivery to the brain.
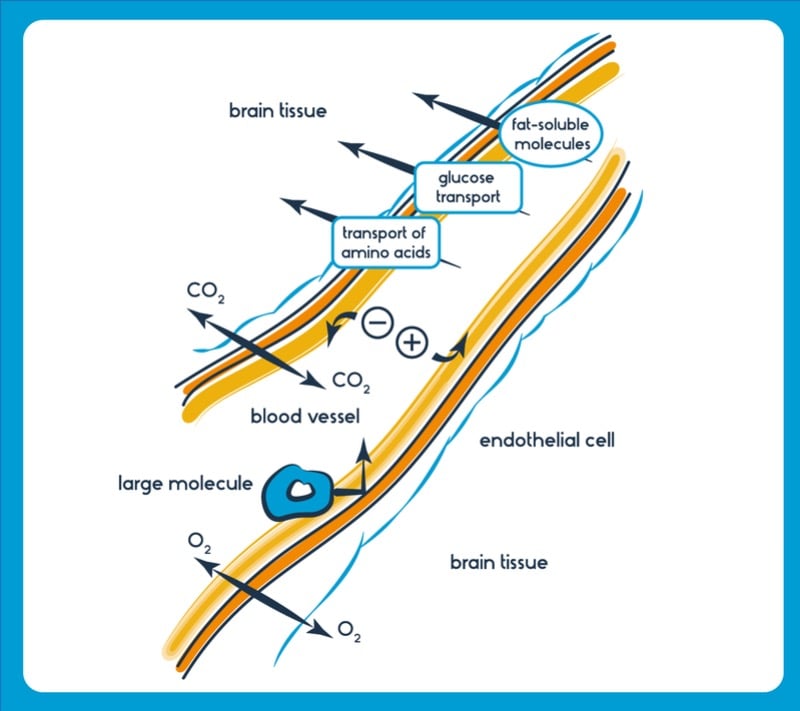
British biotech Ossianix has identified several variable new antigen receptor (VNAR) fragments, now in development for use as blood–brain barrier shuttles. These molecules, derived from shark antibodies, are some of the smallest antibodies known. Ossianix is using them to generate drugs that can enter the brain for the treatment of glioblastoma, multiple sclerosis and pain, amongst other conditions.
VNARs have a unique structure, concentrating the majority of their antigen recognising ability in a notably different site to their human counterparts. “Their mode of binding appears to be different than that of other antibody fragment-based modules,” Frank Walsh, CEO of Ossianix told me. “This allows for recognition of structurally diverse antigens and epitopes buried in the cavities and clefts of proteins that are not normally available to regular antibodies.”
Most recently, Ossianix announced a research collaboration with Danish diabetes giant, Novo Nordisk, to further develop their blood–brain barrier shuttle technology focussing on diabetes and metabolic diseases.
Stowing drugs in exosome bubbles
Over the past 10 years, exosomes have been gaining significant momentum as a drug delivery system. Originally considered cellular packages of junk, exosomes are lipid vesicles just a few nanometres wide loaded with molecular cargo. They are a way cells naturally communicate and exchange molecules.
Spun out from collaborators between the University of Oxford and the Karolinska Institute in 2016, Evox Therapeutics is a UK-based company focused on engineering exosomes for drug delivery across the blood–brain barrier. Currently, Evox is investigating over a dozen brain-targeting molecules that can be expressed on the exosome surface as a means of targeting the blood–brain barrier.
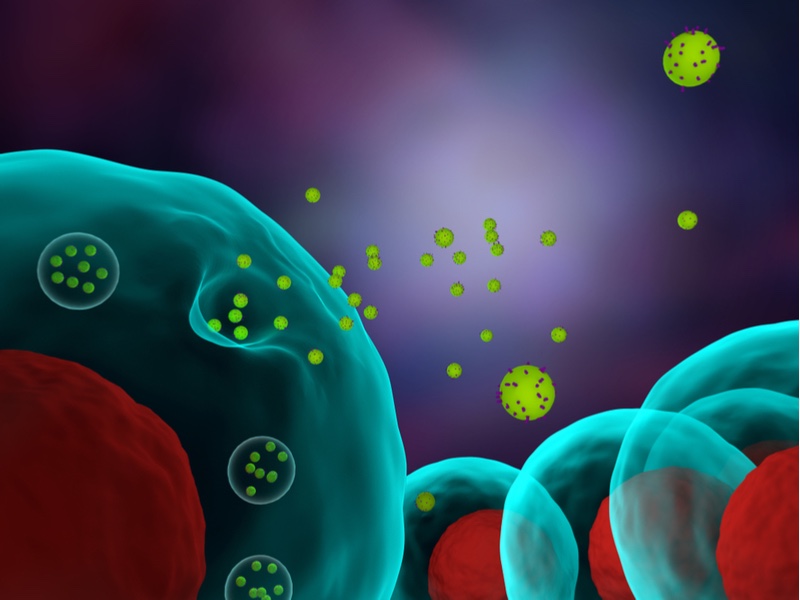
“Exosomes can cross the blood brain barrier at a decent level by themselves, without any particular targeting. But we’ve also shown that if you attach ligands to the surface of these exosomes, it then helps them target the brain, and you can improve the amount that crosses the blood brain barrier by 5- to 10-fold,” Antonin de Fougerolles, CEO of Evox, pointed out.
A significant advantage of exosomes is their safety profile. “If you think about safety, each time you give a blood transfusion, a unit of blood will contain [billions of] exosomes per unit of blood. These transfusions happen about 85 million times a year, and are actually very safe.”
To date, over half a dozen have been conducted in humans showing well tolerated single and repeat dosing. “We see it has great potential with regards to being able to deliver a whole range of drug modalities – proteins, antibodies, small molecules, siRNA, mRNA… we can load any drug class and target these to particular tissues,” he explained.
Working in the same space, ArunA Biomedical, based in Georgia in the USA, is developing an exosome-based treatment for stroke following observations seen in neural stem cells.
“We knew that in a [stroke] injury model, our neural stem cells had a reparative effect but that there was no integration of the cells into the brain. This, combined with a series of in vitro studies, led us to believe that there was something happening beyond the stem cells themselves,” Steve Stice, CEO of ArunA, told me.
Most recently, when it tested its candidate therapy in a mouse stroke model, ArunA showed a decrease in brain atrophy of nearly 35% compared to before treatment, and a 50% reduction in brain tissue loss; results not previously observed in exosome treatment studies. These results were backed up by further work in a pig model of ischemic stroke, preserving neural tissue and function.
Currently, intravenous tissue plasminogen factor is typically used to treat ischemic stroke. However, in order to show worthwhile efficacy, it must be administered within 4.5 hours from the onset of the stroke. Contrasting this to exosome therapy, ArunA’s candidate therapy showed neuroregenerative effects in the mouse model following administration 6–24 hours after stroke onset.
Using ultrasound to open the barrier
It is possible to gently pry open the tight junctions formed by the cells lining the blood–brain barrier to increase drug uptake. CarThera, a company based in France, has designed the SonoCloud device with this in mind.

The idea came from the work of Alexandre Carpentier, a neurosurgeon at the University Hospital Pitié-Salpêtrière in Paris. Typically, the diagnosis of brain tumours involves performing a biopsy, by drilling a small burrhole in the skull and resealing it afterwards. Carpentier’s idea was to place a small implantable device in this hole that, when activated, emits pulsed ultrasound bursts.
When ultrasound is used in conjunction with the injection of micrometer-sized bubbles and carboplatin, a commonly used chemotherapy drug, drug uptake across the blood brain barrier can be increased.
This was revealed in CarThera’s preliminary results of a Phase I/IIa clinical trial for glioblastoma at ASCO 2018 – showing a good safety profile and positive trends in progression free and overall survival.
“The major problem with glioblastoma is that even by doing a resection of the tumor, you have a lot of healthy tissue that is infiltrated by glioblastoma cells. So, the larger area you can reach, the better it is,” said Frédéric Sottilini, CEO of CarThera. In other words, using the SonoCloud, the treatment can be delivered to regions of the brain where cancer cells have spread.
Beyond glioblastoma, the SonoCloud may have a future in treating Alzheimer’s disease. CarThera has an ongoing Phase I study in Alzheimer’s opening the blood brain barrier every two weeks – evaluating the safety of this approach and measuring if there is an effect.
This use of ultrasound may stimulate the immune system to fend off disease. In mouse models of Alzheimer’s disease, the team were able to see a decrease in amyloid plaques and improvement in the animals’ health by using ultrasound alone and carrying out multiple blood brain barrier openings, Sottilini went on to say. CarThera now hopes to take the device’s application wider, and may be ready for the clinic within 3–4 years.
Aside from blood brain barrier shuttles, exosomes and ultrasound, other strategies are also being developed to deliver molecular payloads to the brain without disturbing normal function – including viral vectors, cell-based delivery, nanoparticle carriers, and molecular trojan horses, for example. But it is clear that, for all of these methods, more work must be done before we see the results in the clinic.
However, with increasing research both at the basic level of understanding of the blood–brain barrier, and creative approaches to navigate a route across it, within the next 5–10 years a spectrum of brain treatments will be readily available for patients.
 Gabriel Hoppen is a science writer based in the UK, with a background in Biochemistry from the University of Leeds. You can find him on twitter @gabriel_hoppen.
Gabriel Hoppen is a science writer based in the UK, with a background in Biochemistry from the University of Leeds. You can find him on twitter @gabriel_hoppen.
Images via Shutterstock and CarThera





