Newsletter Signup - Under Article / In Page
"*" indicates required fields
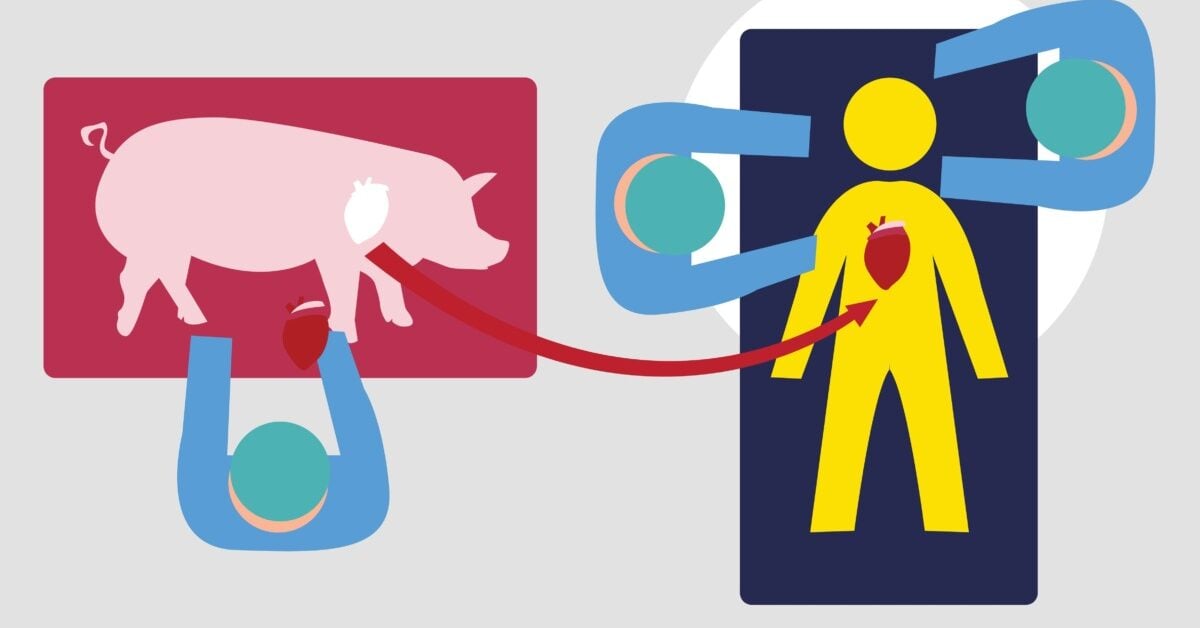
On January 7, 2022, a surgery was performed on David Bennett to give him a new heart. But this was no ordinary transplant surgery. Instead of receiving a heart from a human donor, the 57-year-old, who did not qualify for a traditional transplant, received a pig heart. This surgery marked a historic breakthrough, becoming the first successful transplant of a genetically modified pig heart into a human being – a procedure known as xenotransplantation.
The demand for organ transplants is at an all-time high. Current statistics show that 31,121 transplants have been performed in the U.S. so far this year, with a further 100,000 people on the waiting list – 17 of whom are likely to die today waiting for their transplant. The sheer number of people on the waiting list can be attributed to an organ shortage crisis, not just in the U.S., but on a global scale.
“Recent figures from the federal Organ Procurement and Transplantation Network (OPTN) are alarming: in the U.S., more than 103,000 souls are in dire need of a transplant, and a staggering 88,000 of them are waiting for a kidney,” said Allan Gobbs, managing partner of ATEM Capital, a biotechnology venture firm based in New York, and a member of the Review Committee of the National Cancer Institute, a part of the National Institutes of Health (NIH).
“This translates to patients waiting an average of 3.6 years for a suitable match. The reasons for these lengthy waiting times range from compatibility issues to the sheer lack of available organs. The grim reality is that, daily, 16 to 17 individuals tragically pass away while still on the organ waiting list. Annually, this number grows into the thousands.”
To address the organ shortage crisis, there is one solution in particular that is being considered and heavily researched: xenotransplantation. This refers to the transplantation of living organs, tissues, or cells from one species to another. In this case, the animal organs – pigs are currently thought to be the best source due to similarities in organ size and physiology – would first be genetically modified with human gene insertions and/or pig gene deletions to trick the recipient’s immune system into recognizing the foreign transplant as its own tissue, before being transplanted into the patient.
History of xenotransplantation: varying degrees of success
Many attempts have actually been made over the past 60 years to transplant animal cells, tissues, and organs into humans, all with varying degrees of success. In the 1960s, when kidney transplantation was not broadly practiced due to a lack of donor organs, a surgeon called Keith Reemtsma transplanted chimpanzee kidneys into 13 patients. Although most of the recipients survived for only a few weeks – succumbing to infection and organ rejection – one patient managed to survive for almost nine months, before suddenly dying from what was believed to be an electrolyte disturbance.
Around the same time, other attempts were made to transplant kidneys and livers from animals into humans. Thomas Starzl performed a series of baboon-to-human kidney transplants, and also carried out the first chimpanzee-to-human liver transplant in 1966. And, later on, in 1992, he performed two more liver transplants, using baboons as donors, with one patient surviving for 70 days before succumbing to an overwhelming infection.
Meanwhile, the first heart transplant, using a chimpanzee as a donor, was performed by James Hardy in 1964. But in this instance, the patient died within two hours. In 1983, Len Bailey transplanted a baboon heart into an infant known as Baby Fae, which prolonged her life for 20 days; a record at the time for a heart xenotransplant.
Then came David Bennett.
Revivicor’s gene-edited pig organs provided a historic breakthrough
The advent of cutting-edge gene editing technologies like CRISPR has brought xenotransplantation back into the spotlight, as it allows scientists to address certain issues – such as virologic and immunologic hurdles – that have historically played a role in preventing the advancement of animal-to-human transplantation.
Taking advantage of these advancements, biotech company Revivicor has designed genetically engineered pigs with ten genetic changes; four pig genes have been deleted, while six human ones have been added. This makes the organs more compatible with humans. Bennett, who had end-stage heart failure and was on his deathbed with no other options, received a heart from one of Revivicor’s pigs, courtesy of the Food and Drug Administration’s (FDA) ‘compassionate use’ authorization, allowing experimental treatments for emergency cases.
Following his heart transplant conducted by the University of Maryland School of Medicine (UMSOM), Bennett remained alive for two months before dying of heart failure.
“Our findings on autopsy did not show evidence of rejection,” said Bartley Griffith, professor of surgery and the Thomas E. and Alice Marie Hales distinguished professor in transplantation at UMSOM, in a press release. “Instead, we saw a thickening and later stiffening of the heart muscle leading to diastolic heart failure, which means the heart muscle was not able to relax and fill the heart with blood as it is supposed to.”
It is thought that several factors may have contributed to Bennet’s heart failure, including the use of intravenous immunoglobulin (IVIG), a drug that was given to the patient twice during the second month after the transplant to help prevent rejection and infection. The drug contains antibodies against pig cells that may have interacted with the pig heart, causing a reaction that damaged the heart muscle.
The heart was also found to contain evidence of DNA from a latent pig virus called porcine cytomegalovirus (pCMV) through highly sensitive testing that was first detected several weeks after the surgery, and was later confirmed during an autopsy of the organ. This is something that could also have led to Bennett’s heart failure.
eGenesis: the company on a mission to solve the organ shortage crisis
Cross-species transmission of viruses is one of the biggest risks presented by animal-to-human transplantation.
With this in mind, biotech company eGenesis, which is based in Cambridge, Boston, is attempting to go one step further than Revivicor. Its platform is the only technology of its kind to address both viral risk and cross-species molecular incompatibilities.
“Infectious disease transmission has been an area of critical focus and concern for xenotransplantation since the field has become more established; these concerns drove the establishment of a moratorium on xenotransplantation development activity in the late 1990s,” said Mike Curtis, president and chief executive officer (CEO) of eGenesis.
“eGenesis was founded to leverage CRISPR and other advances in molecular biology to address these concerns by genomically inactivating an endogenous retrovirus that has been a major concern historically. We are unique in advancing donor organs that lack the potential for retroviral disease transmission. Additionally, our organs are produced in state-of-the-art biosecure facilities that employ robust testing and quality control prior to product release to further reduce the risk of adventitious agent transmission to patients.”
The company’s platform is called the eGenesis Genome Engineering and Production (EGEN) platform. This is where the process starts to create genetically modified organs, and it is used to modify the genome to reduce the molecular incompatibilities driving organ rejection and to address viral transmission risk. “These edits included knock out of three genes involved in the synthesis of glycan antigens that contribute to acute organ rejection, insertion of seven human genes to promote long-term organ compatibility and function, and inactivation of the endogenous retrovirus embedded in the porcine genome,” explained Curtis.
The modified cell then undergoes single-cell cloning, before being sequenced for genetic verification. The nucleus of the genetically modified cell is then inserted into an enucleated oocyte, and the resulting embryo is transferred into a surrogate for gestation in one of eGenesis’ state-of-the-art donor production facilities.
The company’s HuCo organs are recovered from resulting donors for evaluation in pre-clinical transplant studies. As eGenesis advances into clinical trials, these organs will be shipped to study sites for evaluation in patients.
“Our vision is to reimagine the future of organ transplantation to address not only the organ shortage crisis, but also variable donor quality and incompatibility associated with the donation of human organs, and ultimately broaden access and serve patients in an efficient and equitable way,” commented Curtis.
What is the future of xenotransplantation?
So, what comes next in the field of xenotransplantation?
Well, despite great strides being made in xenotransplantation in the last few years, research is still very much ongoing, with the procedure currently being tested in deceased individuals. After all, Bennett only lived for two months after his transplant, and it is still not entirely clear why his porcine heart failed, which suggests there is much more to be learned about the process. Having said that, the research seems to be progressing very quickly.
“In addition to demonstration of longer-term survival with organs carrying multiplexed edits made possible by the latest advancements in genome engineering, the field has begun to evaluate edited porcine organs in decedent (or recently deceased) recipients,” said Curtis. “This represents a major step forward toward clinical trials as studies involving decedents enable greater insight into the safety and efficacy of xenogeneic organs in the clinical setting than research in the preclinical setting.”
Much of this research is taking place in universities and medical centers across the U.S.. For example, Gobbs said that NYU Langone Health is at the forefront of this research, particularly in addressing organ rejection.
“I was truly impressed to learn that surgeons at NYU Langone Health had successfully transplanted a genetically engineered pig kidney into a deceased man, which functioned optimally for over 32 days,” he said. “Led by Dr. Robert Montgomery, the July 14, 2023, surgery was the fifth of its kind at NYU Langone. There were no signs of organ rejection. The key to success was a gene “knockout.” Contrary to earlier procedures with multiple genetic changes (10-40), this study indicates that a single-gene modification might suffice. Monitoring will persist for another month to further understand this medical breakthrough, edging closer to potential clinical trials.”
UAB Heersink School of Medicine is also making strides in its research in the field. In a pre-clinical human research model study conducted in February, in a recipient experiencing brain death, researchers from UAB managed to show that genetically modified pig kidneys provided life-sustaining kidney function for the first time in a human.
“What studies demonstrate is that the field has been able to overcome some of the key challenges that have impeded advancement of the field to date such as hyperacute rejection that occurs when the recipient immune system attacks the donor organ, resulting in graft failure,” said Curtis.
“The success of these recent procedures in maintaining functional organs (kidney as well as heart) through the acute phase of transplant illustrates the success of edits to the genome to eliminate the antigens driving acute rejection. Additionally, the longer-term xenotransplants that have been conducted demonstrate the success of additional edits to the genome in modulating the mechanisms that drive longer-term rejection such as complement activation, and coagulation incompatibility.”
What are the main objections to xenotransplantation?
Some still have their doubts about xenotransplantation being the best solution to solving the organ crisis, though.
Dorothee Caminiti, director of bioethics at Markkula Center for Applied Ethics, Santa Clara University, pointed to the risks simply being too high. “Major epidemics have been initiated through cross-species transmission of viruses from animals – wild or domestic – to humans. Yet, according to experts in the field, our understanding of this complex process is still very limited.”
“And even when those risks can be better mitigated against, what other negative and currently unknown consequences will arise from mixing species? And how can we ask patients to give their informed consent in such circumstances? Most of the time, when we’ve decided to mix species in some way, the procedure made them weaker, or even extinguished them. We certainly do not want the same to happen to us, humans.”
Instead, Caminiti believes we should try to find other ways of solving the organ shortage crisis before bringing xenotransplantation into the mainstream. “The best approach, in my opinion, is investing heavily in prevention, keeping people healthier, and providing the patients we could not help with human organs. Another approach to better addressing the organ shortage crisis, for instance, is by changing the opt-in option that most countries currently have into an opt-out system, where organ donation will occur automatically unless a specific request is made before death.”
While Caminiti’s concerns are certainly warranted, using a careful approach like eGenesis’ – in using their platform to try and eliminate cross-species transmission – it is possible that these risks could be preventable, which could pave the way for xenotransplantation to succeed clinically.
And, if that happens, who is to say it cannot become a viable solution to solving the organ shortage crisis?
“From my perspective, xenotransplantation stands out as a revolutionary answer to this urgent dilemma,” said Gobbs.