Newsletter Signup - Under Article / In Page
"*" indicates required fields
The digital transformation of biopharmaceutical process development is known as Bioprocessing 4.0. Using the internet of things, digital biomanufacturing creates a network of data sources, materials, equipment, and industry representatives. This creates a competitive advantage, increases productivity, and boosts the value of biomanufacturing.
We have spoken to Merrilee Whitney, Head of the BioContinuum™ platform at Merck* about the growing interest in Bioprocessing 4.0, how the Covid-19 pandemic has influenced biomanufacturing, and how Merck’s new software suite, Bio4C™, supports the digital transformation of biopharmaceutical process development.
What is driving the growing interest in Bioprocessing 4.0?

Drug manufacturers are faced with complex regulatory requirements, intense cost pressures, a growing number of new product classes, and high development costs. These challenges are being addressed, in part, by an evolution towards the biopharmaceutical manufacturing ‘facility of the future’, which is characterized by intensified, continuous, predictive, and autonomous operations. These are the cornerstones of the Bioprocessing 4.0 evolution and include digitization and automation.
Looking ahead, there is a clear industry movement to leave behind paper-based bioprocessing, data silos, manual process control, and equipment that cannot communicate with each other. In its place will be a manufacturing ecosystem, seamlessly connected via uninterrupted data acquisition and analysis, and characterized by information transparency and decentralized decision making.
Drug manufacturers will be able to control and understand every aspect of their operation and leverage real-time data to boost productivity, increase operational efficiency, and drive growth more effectively and efficiently.
What impact has the Covid-19 pandemic had on biomanufacturing?
The speed at which we’ve seen Covid-19 vaccines be developed and manufactured is remarkable and a testament to both innovative, agile thinking and to the new technologies being applied to address this global challenge.
The industry is conceptualizing next-generation bioprocessing and manufacturing tools and technologies to accelerate the discovery and production of safe and effective life-saving and life-enhancing medicines.
Our Bio4C™ Software Suite is an example of a digital ecosystem of products designed for bioprocessing to empower biomanufacturers to achieve greater speed, flexibility, and quality while reducing risks and costs.
What is the Bio4C™ Software Suite?
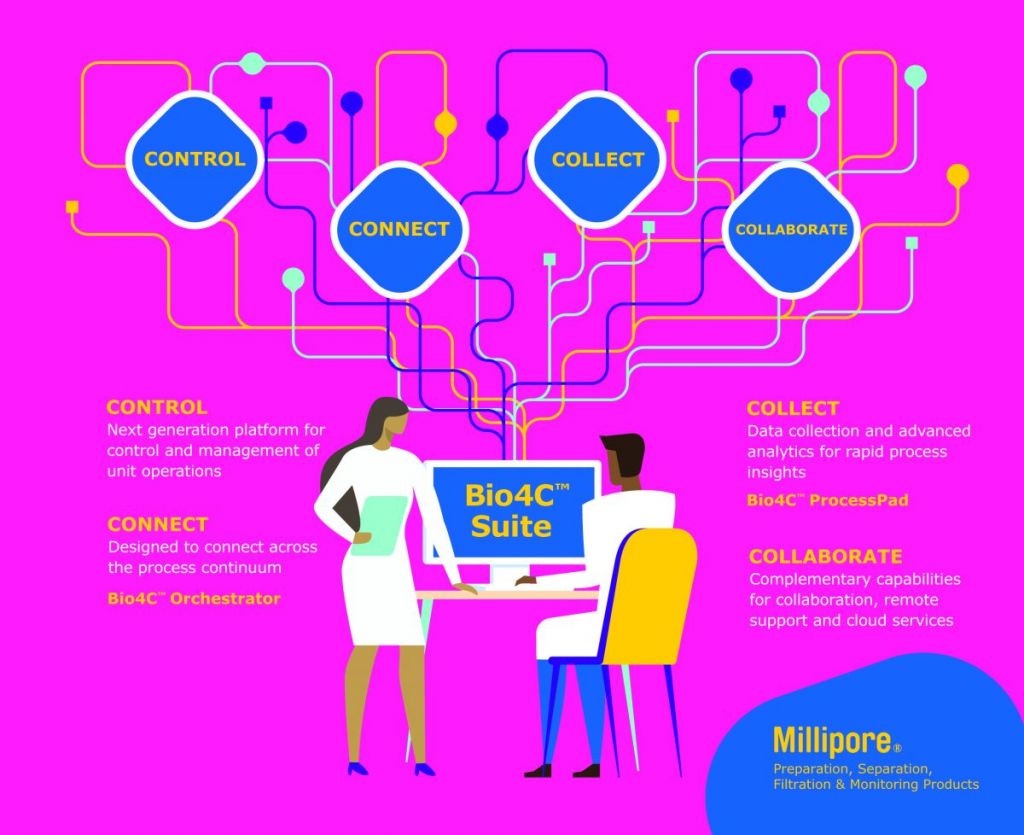
The Bio4C™ Software Suite, which we launched in 2020, comprises several seamlessly compatible, software products. The products provide users remote access and the ability to look across the entire manufacturing process, versus individual operational units.
The first software product we introduced is Bio4C™ ProcessPad. This software allows customers to automatically collect data from disparate sources into a single and unified platform to better understand, improve, and ultimately, optimize the way biotherapeutics are made. This delivers greater process control and insights through a single integrated, browser-based platform for data acquisition, aggregation, and analysis; allowing customers to manage the velocity and volume of complex, disparate electronic and batch record data sources more effectively. It also makes reporting and analyses less time- and labor-intensive.
Our second product is the Bio4C™ Orchestrator which enables users to centrally – and ideally remotely – connect and integrate bioprocess systems and fully digitize their process workflows. It allows the connection of individual skids in an orchestration layer that provides monitoring, visualization, and centralized execution of manual activities in real-time. With this connectivity, users can optimize processes, improve quality and compliance, and accelerate time to market.
Can Bio4C™ integrate existing digital solutions from other providers?
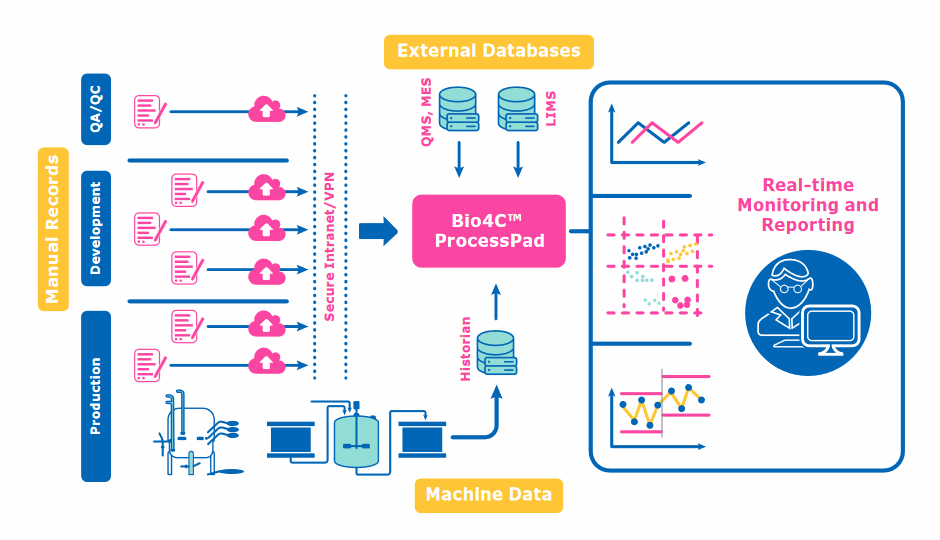
For the Bio4C™ ProcessPad, we can connect to systems or unit operations from any provider, so it is truly agnostic to the equipment on the manufacturing floor. The first release of our Bio4C™ Orchestrator platform works with Merck products and the next release will include the capability to work with systems from other providers.
How does Merck support customers when they order the software?
We start with understanding the problem the customer needs to solve. With the Bio4C™ ProcessPad, we discuss how they manage their disparate data sources, what kind of challenges they face as they aggregate the information, and understand how their processes are defined manually.
We work with the customer to design automated data integration from laboratory information management systems or enterprise resource planning systems and create forms for operators to input manual entries or process observations. We help the customer design dashboards for visualization and reporting and conduct a ‘train the trainer’ session with the key end-users.
For the Bio4C™ Orchestrator, we work with the customer’s automation teams and integrators to plan the implementation of the software. Once we have a detailed implementation strategy and project plan, we work together through the phases of user requirement specifications, design, implementation, validation, and training, followed by going live.
What other software solutions will be part of the Bio4C™ suite?
The Bio4C™ Software Suite aims to address the automation needs of bioprocessing in a holistic manner with solutions for control automation, equipment connectivity, data collection, and collaboration. With this guiding principle, we are building the next generation control platform called Bio4C™ ACE (Application Control Engine) for unit operation automation.
For process analytical technology (PAT), we will be offering the ProCellics™ Raman Analyzer with Bio4C™ PAT Raman Software in 2021, which can measure a broad range of bioprocessing parameters simultaneously, providing real-time monitoring and process control.
The Bio4C™ Customer portal will provide our customers a single web-based interface to track the service-related information for their software and systems products.
How will the landscape of Bioprocessing 4.0 develop in the near future?
Current processing models and approaches have advanced as far as they can go in terms of flexibility, utilization, risk mitigation, and speed to market. If we want to see real step changes in manufacturing costs, efficiencies, and plant utilization now, then we need to look at bioprocessing in a new way – which will involve new technologies and methodologies.
For some in the industry, next-generation bioprocessing means a completely single-use manufacturing facility, while others describe it as a fully connected and fully continuous process.
From my perspective, the goal is the latter. The true definition of next-generation bioprocessing is the collective set of technologies that are needed to deliver higher productivity, increased manufacturing flexibility, increased speed, and reduced risk.
If you are interested in learning more about Merck’s Bio4C™ Software Suite and how it lays the foundation for Bioprocessing 4.0, check out these videos to learn more:
- Bio4C™ ProcessPad – Acquire, Aggregate, and Analyze Your Bioprocessing Data
- Bio4C™ Orchestrator Software Product Overview
- Bio4C™ Orchestrator Software Product Demo
*The life science business of Merck KGaA, Darmstadt, Germany, operates as MilliporeSigma in the US and Canada.
Images via Merck and header image via Shutterstock.com