Newsletter Signup - Under Article / In Page
"*" indicates required fields
Quantoom Biosciences has just launched Ntensify™, the world’s first continuous mRNA production technology that is set to revolutionize the entire mRNA manufacturing workflow.
How it all began
In 2013, two biotech entrepreneurs, José Castillo and Hugues Bultot, founded Univercells with one mission in mind: make biologics for all. Their belief in the potential of technology to break down the barriers to large-scale production of biologics drove their ambition to make these medicines accessible to people who couldn’t afford them.
Fast forward to 2021, and the world was facing a global health crisis with the outbreak of Covid-19. In the search for solutions, RNA-based vaccines emerged as a promising option. However, the production of RNA vaccines at scale posed significant challenges. This prompted José Castillo to establish Quantoom Biosciences (‘Quantoom’) in June 2021, with the goal of revolutionizing the production of mRNA. With the promise of delivering the highest quality mRNA at a lower cost, Quantoom’s platform holds the potential to democratize access to RNA-based therapeutics, including vaccines.
Less than two years after its inception, Quantoom’s Production Technology Line, Ntensify, is now ready for commercialization. This groundbreaking platform holds the potential to transform the production of mRNA-based therapeutics and make them more accessible to patients worldwide.
A recent webinar hosted by Quantoom provides an opportunity to learn more about the technology and the benefits behind Ntensify.
The current barriers in mRNA vaccine development and how Ntensify overcomes these
There are three major barriers to the production of mRNA:
- The design of an optimal mRNA sequence is complex and requires a significant amount of time to identify and select the most promising mRNA candidates.
- The development of a suitable production process is complicated and involves the use of specific reagents that must be carefully selected and tested to ensure high performance.
- A demanding scale-up and process validation is necessary for both clinical trials and commercial production. This involves ensuring that the manufacturing process can be scaled up effectively without compromising the quality of the final product.
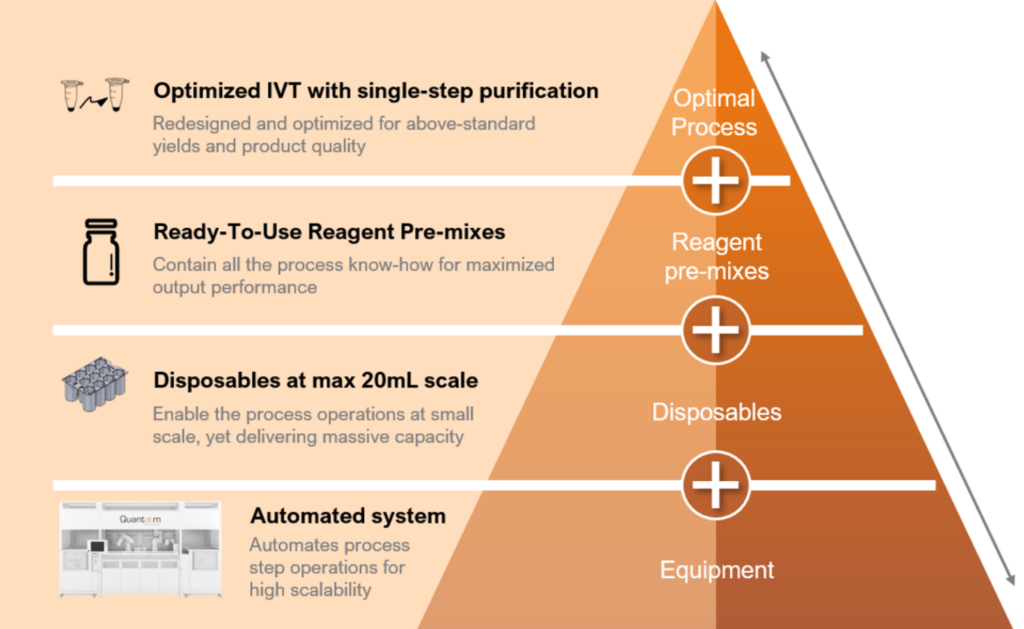
Ntensify is low footprint automated production technology able to synthesize and purify mRNAs from research scale up to production scale. When using Quantoom’s reagents, the yield is maximized without compromising on quality or cost. The input material is DNA, and the output is ready-to-formulate RNA. Ntensify also provides customers with optimized processes, ready-to-use disposables, and reagents to aid in the development of mRNA vaccines or therapeutics.
The redesigned and optimal in-vitro transcription (IVT) process was co-developed with eTheRNA, allowing continuous production in a sequential mode of 20mL batches. This approach avoids scaling up, which is known to bring related risks. Impurities are limited, reducing the purification process to a single step. Moreover, the process is mRNA construct-agnostic, making it a versatile solution.
To ensure optimal performance, Quantoom offers ready-to-use reagent mixes that are pre-prepared at the correct concentrations, volumes, and ratios, in the right buffers. Disposables have been designed so that customers can benefit from a hassle-free process with improved efficiency.
Finally, Ntensify has a very small footprint, replacing entire steps in the manufacturing process, and incorporating disposables to provide an optimized process.
Ntensify is available as a suite of three models to meet different needs at each stage of the mRNA production journey. The Ntensify mini is ideal for research and development, screening up to 192 constructs or for small-scale production (mg) for one particular mRNA construct required for in-vivo studies. The Ntensify midi is suitable for clinical trials and mid-capacity GMP-grade production, providing 1-5g of RNA in a day and 7 million doses in a year (at 50 µg/dose). It is GMP-compliant and will soon be upgradable to the Ntensify maxi which offers drastically increased continuous production of more than 50 million doses per year (at 50 µg/dose).

The benefits of the Ntensify Technology Line
Ntensify simplifies mRNA programs, which has several benefits. Firstly, it removes risks by delivering above-standard performances for yield and quality, which has been proven on mRNAs (1,000 – 4,000 nucleotides) and saRNA (up to 11,000 nucleotides). Secondly, it accelerates time to market by enabling faster candidate screening and µg to multi-kg mRNA production without needing to optimize the process. Finally, Ntensify offers cost-efficiency, reducing capital expenditure as well as operational costs on reagents.
Case study
Using Ntensify to produce 50 million doses of mRNA vaccines (50 µg/ dose/ year) compared to a 40L conventional approach:
Ntensify maxi can produce 50 million doses of mRNA vaccines per year, in just 42 batches:
- Ntensify maxi requires 3-fold lower IVT volumes (13L instead of 40L) for the same end-product volume, resulting in a reduction of reagents required (capping reagent, enzymes, media) and needs only one purification step. This optimized process leads to significant cost savings.
- By producing at 20 mL and operating in sequential continuous mode, Ntensify maxi can save 15 months and ~7.5 million euros in materials and FTE costs. There’s no need for time-consuming scale-up from benchwork.
- Ntensify’s automated IVT and single-step purification result in a lower batch failure rate and > 6 million euros of savings in annual operating costs.
- Ntensify’s compact design allows it to fit into one 30 sqm container. A smaller vaccine facility saves an additional 4 million euros in CAPEX, by eliminating the need for Media and Buffer preparation rooms and requiring less warehouse space. Overall, the production of 50 million doses of mRNA vaccine can be accomplished in a 300 sqm facility, rather than an 800 sqm facility.
DNA is a critical starting component for the manufacture of RNA. Quantoom has addressed this need by providing its customers who acquire Ntensify with a DNA on-demand service. This service includes plasmid DNA engineering and amplification parameters screening, resulting in purified DNA for use in the Ntensify systems. The DNA can be provided in less than 6 weeks at scales ranging from µg to 70 mg and is provided with a certificate of analysis, including NGS, DNA integrity, purity, endotoxin assay, and more.
Ntensify has the potential to benefit a variety of companies in the field of vaccine development and production in both human and animal health. Biopharma companies already engaged in vaccine development may find that Ntensify provides an edge over their competitors, allowing them to accelerate their research and development efforts. Contract development and manufacturing organizations (CDMOs) may also find that the technology allows them to differentiate their services.
Similarly, biotechnology companies that are seeking to bring the first promising vaccine candidate to market may be able to speed up their development process.
Quantoom stands out from other companies in the field as the only company able to supply the equipment, plastic disposables, reagents, and related DNA services for continuous mRNA production from µg to kg in a single small-footprint instrument.
Get in touch with Quantoom at bd@quantoom.com and join our first customers to leapfrog competitors in your industry.
Here is the link to the webinar and click here to discover more about Ntensify.
Images courtesy: Quantoom Biosciences, Shutterstock