Newsletter Signup - Under Article / In Page
"*" indicates required fields
Antibody-drug conjugates (ADCs) represent an innovative class of potent anti-cancer agents. They combine the specificity of a monoclonal antibody with the potency of a cytotoxic drug, enabling targeted killing of cancer cells with lower off-target effects. Over time, ADCs have evolved to high complexity and variability, posing increasing challenges in product characterization and quality control that must be addressed to advance their use in the clinic.
The first ADC received accelerated regulatory approval in 2000 for treating acute myeloid leukemia. It consisted of a cancer cell-specific monoclonal antibody (mAb) and a cytotoxic antibiotic as the drug component or payload.
Today, ADC payloads cover a broad chemical space, ranging from small molecules and peptides to DNA and RNA oligos. This diversity reflects the many options for eliminating cancer cells and the potential for addressing diseases beyond oncology, including infections and autoimmune disorders.
Clinical advances and molecular technologies have transformed antibody-drug conjugates into “anything-drug conjugates” as mAbs have been joined by a plethora of other carriers, including bispecific antibodies, antibody fragments, recombinant proteins, and even virus-like particles.
Linker molecules and conjugation chemistries have co-evolved to provide a broad range of coupling reaction options that increase the complexity of the manufacturing process and the number of intermediates that must be characterized during quality control procedures.The growing diversity of ADCs has resulted in challenges in product characterization that must be understood and considered to mitigate delays in development efforts and market introduction. Contract development and manufacturing organizations (CDMOs) are playing a crucial role in gaining experience and supporting the industry.
Table of contents
The challenges of ADC characterization
The primary goal of ADC characterization is to identify all critical quality attributes. The resulting specifications must be established and adequately documented for regulatory submissions as the ADC moves into clinical trials (Investigational New Drug, or IND, application) and, ultimately, to market (Biologics License Application, or BLA).
In particular, a Quality Target Product Profile (QTPP) must be created to develop a quality control strategy for the product and define measurable parameters and requirements that need to be met during the manufacturing and quality control processes.
Due to the complex structure of ADCs, the control strategy is multifaceted, and more analytical specifications are required than for mAbs or small molecules alone. In addition, ADC characterization protocols must keep pace with the growing variety of conjugates, increasing the challenge for the pharmaceutical and biotech industries to meet regulatory requirements.
“Product characterization activities must begin in preclinical development and continue throughout the entire development process to marketing introduction,” said Deanna Hunt, Senior Director of Analytical and Formulation Sciences Business Development at KBI Biopharma, a global CDMO with extensive experience in ADC characterization.
ADC characterization techniques
KBI Biopharma has established a comprehensive workflow of characterization methods to elucidate the critical structural components of ADCs. For instance, the primary and higher-order (secondary and tertiary) structures are assessed to ensure the protein is correctly synthesized and folded with appropriate configurational and conformational stability.
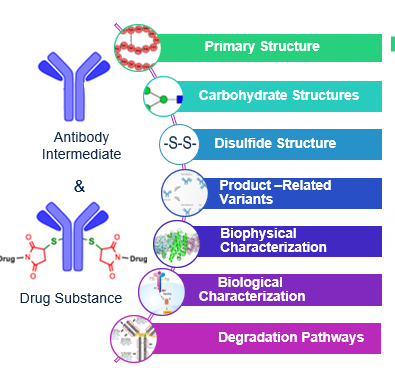
Furthermore, conjugation variants, post-translational modifications, and disulfide bonds and variants are evaluated to elucidate product attributes and to enable an analytical control strategy. Impurities resulting from the conjugation process are identified and investigated. Information on the biological mechanisms and degradation pathways of the ADC is also collected and documented.
The methods used for ADC characterization must be developed and qualified to ensure that they work with both the carrier protein before conjugation and the ADC after conjugation. One example is Differential Scanning Calorimetry (DSC), which is used to evaluate changes in conformational stability upon conjugation of the payload to a carrier protein. Thermal transitions of the different regions of a monoclonal antibody – the Fab, Fc, and hinge regions – may be used to evaluate the comparability of the molecular tertiary structure before and after conjugation. As a stability-indicating method, DSC may be used to evaluate the degradation rates and relative stability of conjugated and unconjugated products under stress conditions, such as light, temperature, and pH.
Elucidating the higher-order structure is essential when evaluating potential critical quality attributes to include in the QTPP. Due to increased hydrophobicity, ADCs are generally more prone to aggregation, which may alter potency and increase the potential for immunogenicity. Another important safety and efficacy-related critical quality attribute is the level of free drug in the drug product, particularly for toxic payloads. Some ADCs are susceptible to deconjugation of the cytotoxin, which can lead to off-target effects.
Experience shows that product characterization protocols should include orthogonal methods for selected parameters to assure that the resulting data are complete and accurate and to aid in drawing conclusions from the data. For example, higher-order structure is determined by correlating data from size-exclusion chromatography (SEC) and ultracentrifugation experiments to ensure no degradation or aggregation events are missed due to technological limitations.
“As a CDMO, we provide expertise in the complexities of ADC products and access to cutting-edge analytical characterization techniques to ensure that our customers have a comprehensive understanding of their drug product attributes to present in regulatory filings.”
“We enable an effective control strategy for our customers’ manufacturing activities, reducing the potential for surprises found late in development or during regulatory review, and increasing the likelihood of a successful launch,” explained Hunt.
Documentation and regulatory filing for ADCs
Regulatory agencies treat ADCs with cytotoxic payloads as both chemotherapeutics and biologics, increasing documentation and paperwork. Therefore, developers must take extra care to establish a testing protocol that collects and connects all the analytical data points needed for the filing documents. Any misinterpretation and incorrect or missing data can create substantial risks.
On the other hand, not all analytical steps are required for each manufacturing intermediate. The modular structure of ADCs may allow combining the data obtained for a specific carrier protein used with different payloads.
“Our team selects the data needed to manage our customer’s budget and time constraints. We work together as one site to perform the complete product characterization,” Hunt noted.
“We link all the data, provide all the necessary data analyses, and can contribute to or even write a draft of the relevant filing section. Customers only need to format the text and edit it to their tone to make the necessary connections to other sections,” she added.
Partnering with a CDMO for ADC characterization
Many pharmaceutical and biotechnology companies prefer to work with a CDMO rather than trying to build the expertise and hardware in-house. When choosing a CDMO to develop an ADC, it is critical to select a partner with extensive experience and specialized capabilities to handle product and process characterization throughout the entire process—from cell line development to commercial-scale manufacturing.
KBI Biopharma is an industry-trusted expert in complex molecules such as ADCs and offers a range of innovative technologies that give customers confidence in their drug development. The company also has expertise in bioconjugation, having developed processes for more than ten bioconjugate programs using carrier proteins expressed in mammalian cell lines and microbial systems.
Successfully completed projects include preclinical, clinical, and commercial products, from pilot to commercial-scale manufacturing. Leveraging a strategic partnership with Argonaut, KBI Biopharma’s end-to-end offering also includes fill-finish capabilities, empowering seamless drug product manufacturing. In addition, the CDMO is expanding its capabilities to include small-scale bioconjugation of hyper-potent (and highly toxic) payload molecules.
Customers typically order the complete package of ADC manufacturing, characterization, QC strategy development, and documentation. Recently, the new KBI Biopharma Analytics Portal was launched for those interested in standalone services, including characterization services for ADCs or other products.
“Through the Analytics Portal, customers can quickly connect with our scientific experts and start their analytical work without undergoing a lengthy service purchasing process,” highlighted Hunt. “They can request quotes, place orders, monitor the shipment of their samples, track progress, and download analytical data files.”
Realizing the potential of ADCs through rigorous characterization
ADCs have significant therapeutic potential, but establishing testing methods for product characterization is often challenging and cumbersome. Proper planning at all stages of development can help mitigate delays and meet regulatory timelines.
A collaborative, experienced analytical team with the right skills, tools, and data integrity is essential for efficiency and success. KBI Biopharma offers a “right the first time approach,” selecting appropriate methods from the preclinical phase to market introduction and providing a complete package of data generation, analysis, and reporting.
Learn more about ADC characterization at KBI Biopharma
Images Courtesy: KBI Biopharma






