Protein inhibitors are valuable tools in understanding molecular mechanisms of many diseases, including cancer or autoimmune disorders. However, with the overwhelming complexity of cellular pathways, researchers often face challenges in identifying the right protein inhibitors to address their specific scientific questions. A new online resource can help.
Cellular pathways form an interlinked, tightly regulated biological network of extreme intricacy. Together, they balance the biological systems of our bodies, support embryonic development, and protect us from internal and external threats.
Many diseases are characterized by up- or downregulation of individual biomolecules within one or several cellular pathways, making them attractive targets for biomedical research. The role of a specific protein is often studied by inhibiting its activity and exploring the downstream effects on the cell.
Table of contents
Protein inhibitors: Advantages and challenges
Protein inhibitors are typically small chemical molecules that are easy to apply to cell cultures or tissues. Their effects on the target or other proteins in the cellular pathway can be studied using suitable antibodies following well-established laboratory procedures.
“Inhibitors can be highly specific for their target protein or pathways, allowing researchers to selectively manipulate a particular process without affecting others. By observing the effects of inhibition, researchers can gain insights into the functions of specific molecules or pathways in various biological processes,” explained Jennifer Chung, Director of Sales Operations at Santa Cruz Biotechnology.
On the other hand, inhibitors are not always perfectly specific, risking unintended off-target effects, misleading results, and complicated interpretations of experimental data. Inhibitors may also exhibit incomplete inhibition, limiting their use in experiments.
To address these challenges, researchers at Santa Cruz Biotechnology are conducting a project to identify suitable protein inhibitors through literature research and wet lab experiments, and share their results through a freely accessible online resource.
Demonstrating the power of protein inhibitors: Cdc7
In one example, the research team studied the effect of the Cdc7/Cdk9 inhibitor molecule on Cdc7 (cell division cycle 7), a protein kinase involved in DNA replication. In certain tumors, Cdc7 has been shown to be overexpressed to enable rapid cell division.
In reverse, inhibiting Cdc7 is believed to reduce the rate of DNA replication, eventually leading to programmed cell death, or apoptosis.
Apoptosis is characterized by a high activity of dedicated proteases called caspases. Poly ADP-ribose polymerase-1 (PARP-1) is a well-described substrate of caspases. Hence, the formation of PARP-1 cleavage fragments is considered a hallmark of apoptosis.
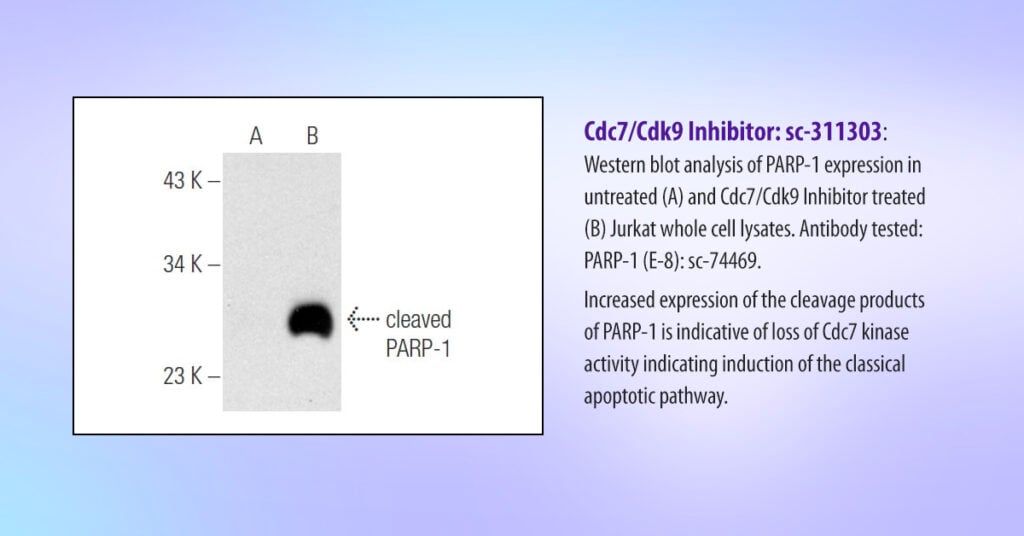
When the Cdc7/Cdk9 inhibitor was applied to Jurkat whole-cell lysates, the scientists at Santa Cruz Biotechnology were able to detect cleaved PARP-1 using specific antibodies (see Figure 1), indicating apoptosis caused by loss of Cdc7 activity.
“Our results confirmed the effectiveness of the Cdc7/Cdk9 inhibitor on Cdc7 described in the literature. In addition, the molecule was also reported to have good selectivity over a panel of 31 other kinases, reducing toxicity and ambiguity in scientific results.”
Studying the Raf/MEK/ERK pathway with protein inhibitors
The Raf/MEK/ERK pathway has an essential function in cell cycle regulation, cell differentiation, and apoptosis. Also, the pathway is involved in resistance towards chemotherapy, stressing its relevance in drug discovery.
Scientists at Santa Cruz Biotechnology tested the Raf kinase V inhibitor for its ability to inhibit the activity of the Raf (Rapid Accelerated Fibrosarcoma) protein family. In particular, studies demonstrated a high affinity of this inhibitor to Raf mutations commonly observed in tumors (the V600E mutation of B-Raf and the Y340D and Y341D mutations of C-Raf-1).
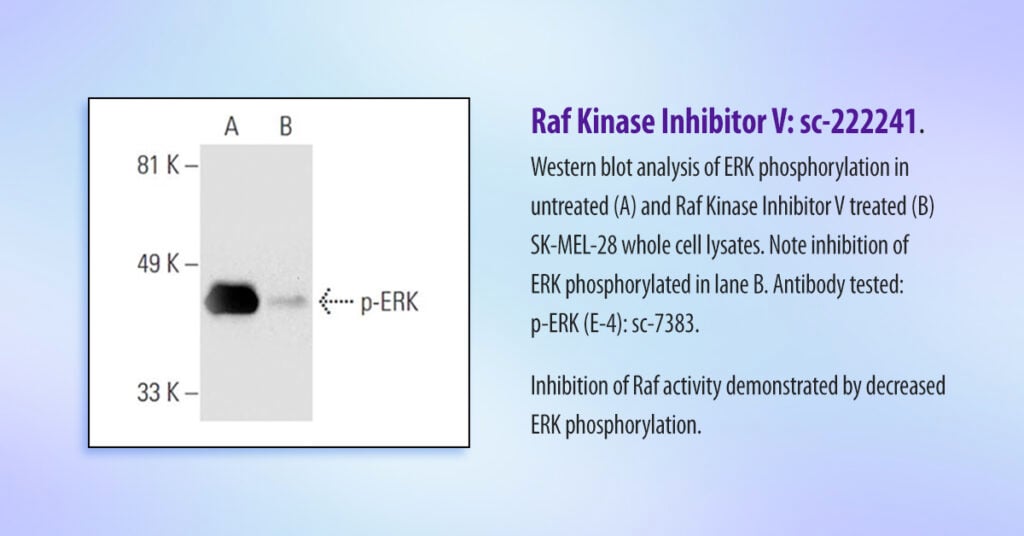
Raf becomes activated by external signals recognized by dedicated receptors on the cell surface. Once activated, Raf activates MEK, which in turn phosphorylates ERK. Finally, phosphorylated ERK interacts with multiple proteins regulating genes involved in cell proliferation and apoptosis.
In their experiment, researchers used a specific antibody to demonstrate that levels of phosphorylated ERK were significantly reduced as a result of Raf inhibition (Figure 2).
“Our Western blot analysis strongly suggests that Raf Kinase Inhibitor V effectively inhibits ERK phosphorylation, a critical event in the Raf/MEK/ERK signaling pathway,” explained Chung.
A complete toolbox to explore cellular pathways
Protein inhibitors are just one product category at Santa Cruz Biotechnology. Antibodies, gene silencing and editing tools, and corresponding buffer components are available in a one-stop-shop that saves researchers time identifying and sourcing the right products.
“We are happy to support the research community with a broad, robust, and easy-to-use application toolbox for studying cellular pathways,” concluded Chappell-Ybarra.
Visit the Santa Cruz Biotechnology overview on protein inhibitors to learn more.
Disclaimer: All Santa Cruz Biotechnology products are for research purposes only and not suitable for use in diagnostics or therapeutics.
Images courtesy: Santa Cruz Biotechnology