Newsletter Signup - Under Article / In Page
"*" indicates required fields
Next-generation proteomics has a vital role to play in the search for new biomarkers of immunotherapy response. Recent studies in melanoma presented at the European Society for Medical Oncology (ESMO) Congress highlight how these insights have the potential to transform cancer care.
The recent success of immunotherapies that manipulate the patient’s immune system to recognize and destroy cancer cells has been a game-changer in oncology. Leading the pack are inhibitors of the PD-1/PD-L1 axis, with other targets such as CTLA4 also rising to prominence.
But while these treatments have been transformational for some patients and diseases, most notably for lung cancer and melanoma they do not work for all. Currently, up to one in five patients achieves lasting results from immunotherapy and there can be significant side effects from the treatment.
Maximizing benefits from immunotherapy
Attention is now focusing on the search for effective biomarkers that can predict response to immunotherapy and the likelihood of serious side effects, in order to support clinicians to choose the most appropriate treatment strategy for their patients and maximize the benefits from these potentially life-saving therapies.
At the recent European Society for Medical Oncology (ESMO) Congress, the world’s leading cancer researchers came together with medical oncologists to discuss the latest progress in understanding and treating cancer.
As might be expected, immunotherapy was high on the agenda, along with a growing role for next-generation proteomics in the quest to find novel biomarkers of response, alongside conventional genomic, transcriptomic, and immunohistochemical approaches.
Combining proteomics and immunochemistry to discover and validate novel biomarkers for immunotherapy response
One study presented by Professor Mitchell Levesque from the University of Zurich Hospital Department of Dermatology, in collaboration with Biognosys and NeoGenomics Laboratories, reported on the use of next-generation proteomics to find a new marker of treatment response in melanoma.

To better understand why some people are resistant to PD-1 therapy, tumor samples were obtained from 24 patients classified either as responders or non-responders following 3 months of treatment. Samples were analyzed using the Biognosys TrueDiscovery™ platform, revealing 76 proteins that were significantly different in the tumor samples from responding or non-responding patients.
“We were particularly interested in PAK4 or p21-activated kinase 4 – an important signaling molecule that is involved in cytoskeleton remodeling, metastasis and growth,” explains Anna Junker-Jensen, PhD, director of scientific affairs at NeoGenomics. “This was then validated using immunofluorescence technology, which also showed PAK4 to be elevated in samples from non-responders and was able to spatially resolve which cells in the tumor express the protein.”
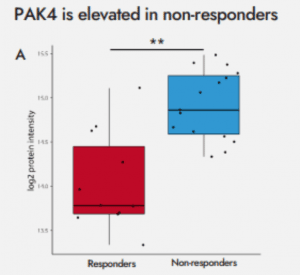
Combining proteomics with transcriptomics for a more comprehensive picture of immunotherapy biomarkers
Another study led by Prof. Dr. Paolo Ascierto, director of the unit of melanoma, Cancer Immunotherapy and Innovative Therapy at the National Tumor Institute Fondazione G. Pascale in Naples, Italy, showed how proteomics could be combined with advanced transcriptomics to uncover additional markers of immunotherapy response.
Using samples from a set of 22 melanoma patients given anti-PD1 therapy as first line treatment, 13 of whom responded to treatment and nine who did not, the team conducted deep proteome profiling using Biognosys’ TrueDiscovery platform and Spectronaut™ software together with RNA transcriptomic analysis.
8,536 proteins and 770 RNAs were analyzed from each sample, with 419 genes identified for which both proteomic and transcriptomic data were available. While many genes, such as the transcriptional activator STAT2, showed similar patterns of up- or down-regulation of protein and RNA levels in responders versus non-responders, there were a few notable exceptions.
Two proteins – MARCO and OAS1 – showed significantly higher protein expression levels but lower RNA levels in the responder group.
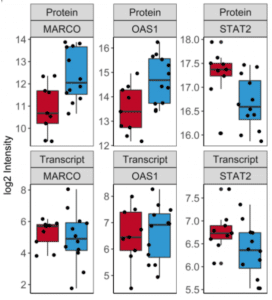

“This unexpected discovery suggests that transcriptomics is not sufficient to identify novel biomarkers of immunotherapy response, and that it should be combined with proteomics to provide a more comprehensive picture of what’s happening within tumors during response or resistance to therapy,” Kristina Beeler, PhD, chief business officer at Biognosys says.
Unlocking the power of proteomics for immunotherapy
These studies demonstrate the power of next-generation proteomics technology to uncover novel biomarkers of response to immunotherapy, either alone or in combination with other approaches such as transcriptomics or immunohistochemistry. The functional insights gained from proteomics can also help researchers to understand the mechanisms of resistance and design new treatments to overcome them.
Unlike purely genomic or transcriptomic approaches, proteomics can reveal the full picture of what’s going on within the body. And only mass spectrometry (MS) proteomics – which analyzes all the proteins present in a sample without the need for antibodies or labeling – has the sensitivity and specificity to go deep within the proteome to reveal novel biomarkers that might be missed by other methods.
Developed by proteomics pioneers Biognosys, TrueDiscovery™ is a high-throughput mass spectrometry platform based on data-independent acquisition (DIA), which is capable of identifying and quantifying thousands of proteins across the whole proteome in an unbiased way.
Delivered through Biognosys’ state-of-the-art GLP-certified and GCP-compliant facility in Switzerland, the TrueDiscovery workflow and resulting data is also highly reproducible between samples and timepoints, making it ideal for clinical biomarker discovery and validation.
Moving forward, the proteins identified here could potentially be used in combination with other biomarkers through Biognosys’ TrueSignature™ custom clinical biomarker panels to stratify patients for clinical trials.
As the era of immunotherapy continues to accelerate, proteomics has a vital part to play in the discovery and validation of clinical biomarkers. The latest technologies offer high-throughput analysis at scale, delivering unbiased insights across the whole proteome to shape the use of these powerful treatments, with the ultimate aim of guiding treatment decisions and delivering better care.
Get in touch with the expert team at Biognosys to find out how their next-generation proteomics solutions can accelerate your immunotherapy research.
Images courtesy of Biognosys.