Newsletter Signup - Under Article / In Page
"*" indicates required fields
A critical step during the production of adeno-associated virus (AAV) vectors is the separation of full and empty capsids during downstream processing. Empty capsids are considered a product-related impurity that, according to US Food and Drug Administration (FDA) guidance, should be characterized and monitored as they may impact product safety and efficacy. The FDA has not yet set a threshold value for the appropriate percentage of full capsids as the clinical impact of empty or partially filled capsids is not sufficiently defined.
The acceptable level of full capsids is typically based on clinical experience. As such, the goal for manufacturers should be to maintain a consistent percentage of full capsids across clinical phases, with an emphasis on robust preclinical and clinical trial designs to understand patients’ tolerance of varying levels of purity.
The similar characteristics of empty and full capsids make their separation quite challenging; full capsids feature only a slightly lower isoelectric point (pI) and a slightly higher density compared to the empty capsids. This article describes strategies for the analysis and separation of empty capsids.
Separation of AAV full capsids from empty and partially filled
Cesium chloride and iodixanol-based ultracentrifugation are commonly used for separation of empty and filled capsids for small-scale preparations and first-generation processes. In larger-scale manufacturing, however, a chromatography-based approach is more desirable due to its scalability.
Anion exchange chromatography has been widely explored for the separation of full and empty or partially filled capsids, due to its scalability, high throughput, and reduced sample preparation. Even with pre-existing process knowledge, time-consuming process development work is needed to optimize parameters such as salts and process additives. A further complication is that AAV can show aggregation under certain process conditions.
Creating a templated approach for capture and separation of capsids
While current approaches for single serotype affinity purification enable better process economics, higher yield, and higher impurity reduction, they are only applicable for a specific serotype. A universal approach for separation of full and empty or partially full capsids without the use of affinity would also offer greater process flexibility.
We are currently developing novel membrane absorbers in collaboration with leading academic experts for affinity capture of AAV capsids. Chromatography membranes have high volumetric throughput due to pore sizes in the 0.5–5 µm range and low bed heights attained with stacks of 3–16 membrane layers. Gel-layer structures are surface coatings that help to boost capacity for large viral vector molecules; coatings are very thin to decrease diffusion distances and preserve mostly convective mass movement. As such, this technique is not limited by diffusion and binding capacities and is not affected by flow rate.
Changing the stationary phase in this manner offers many benefits, including higher flow rates of up to 10 MV/minute. This enables processing over larger sample volumes while reducing buffer consumption. Because convective flow behavior is feasible with larger pore diameters, mass transfer constraints are reduced. Excellent binding efficacy is enabled by direct access to functional groups and high flow rates.
A universal manufacturing template for AAV not only includes a universal capture step, but it also requires a universal approach to separate full from empty capsids. Both resins and membrane absorbers offer potential for empty-full separation. Small resin particles based on anion exchange chemistry, such as Fractogel® resins, hold promise when applied to a hybrid gradient in finding the balance between process yield and separation efficiency for full and empty capsids. These resins can potentially be used in a multi-column approach to further improve efficiency and productivity.
The use of membranes, however, offers much greater flexibility and potentially greater productivity when compared to chromatography resins. For example, Natrix® CH membranes, with a unique multi-modal chemistry that includes sulfonic acid and butyl groups, can be used for capture of AAV capsids. With further optimization, it may be possible to separate empty from full capsids in a single step.
Analytical characterization of the AAV empty/full capsid ratio
Several analytical approaches are available to measure the empty/full capsid ratio, including UV spectroscopy, which uses an A260 to A280 ratio to determine empty/full capsid ratio. While this method is simple, it is highly sensitive to the presence of components that absorb light at either 260 or 280 nm. A combination of orthogonal approaches such as quantitative PCR (qPCR) and ELISA is preferable.
Anion exchange chromatography is commonly used for determination of the empty/full capsid ratio based on differences in surface charges. Figure 1 shows a separation of empty and full AAV8 capsids. It is important to note that this method is serotype dependent, which means that a serotype specific method must be developed for each serotype.
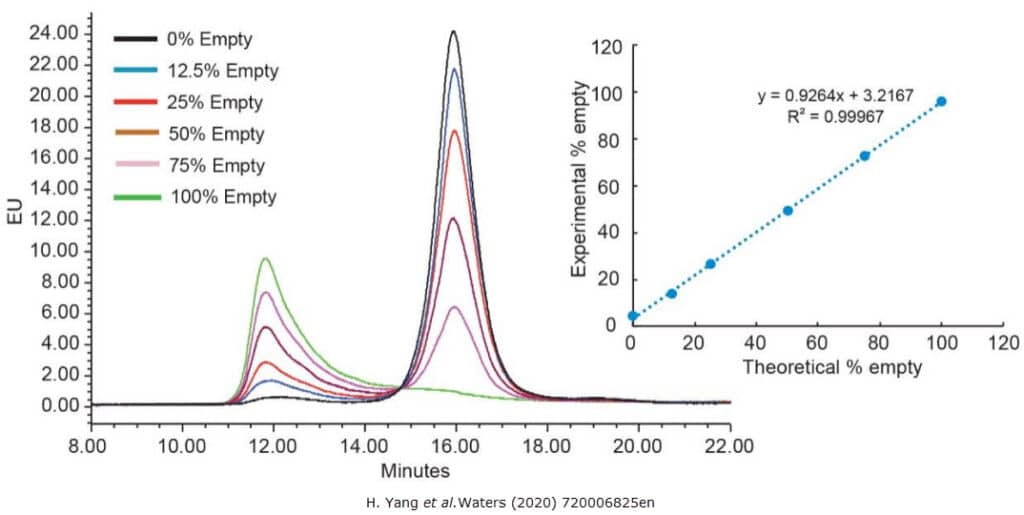
Analytical ultracentrifugation is a gold standard method for determining the empty/full AAV capsid ratio. While this method is stereotype independent, it is more complex to execute and requires higher sample volume than the anion exchange chromatography method.
Novel methods that hold promise for empty/full capsid analysis include size exclusion chromatography and anion exchange chromatography with multi-angle light scattering. These methods offer the potential to be a single approach for all applications, providing information on the full/empty ratio, capsid titer, and level of at-line aggregation.
Conclusion
Due to their similar surface properties, separation of empty and full AAV capsids is especially challenging. Effective purification requires exploration of alternative approaches, which differ from traditional purification methods. Moving to templated chromatography processes is desirable, although this will likely require a case-by-case approach depending on the complexity of separation required for new serotypes. As the AAV industry continues to grow, tools such as these can be implemented for improved purification of full and empty capsids.
To learn more about empty-full separation, click here.
Disclaimer: Fractogel® resins may not be used in commercial manufacturing processes for the separation of empty and full AAV capsids, such AAV capsids are intended for human therapeutic use and which use has received regulatory authority approval for commercial sale, without prior consent by the owner of EP 2 277 996 (Genzyme Corporation, Cambridge, MA) and/or US 8,137,948 (Genzyme Corporation, Framingham, MA) and/or US 9,528,126 (Genzyme Corporation, Cambridge, MA). Purchase constitutes an agreement by the purchaser that the product may not be used in commercial manufacturing processes for the separation of empty and full AAV capsids, such AAV capsids are intended for human therapeutic use and which use has received regulatory authority approval for commercial sale.
Images courtesy: Merck, Shutterstock