Newsletter Signup - Under Article / In Page
"*" indicates required fields
Navigating the complexity of the industry can be highly challenging for early-stage biotechs focusing on biological drug development. Once they have a robust strategy in place and have chosen a suitable partner, biotechs face other hurdles in upstream process development, manufacturing, IND filing, and regulatory processes.
Discover how to address challenges and manage risks throughout biological drug development. Download the report today!
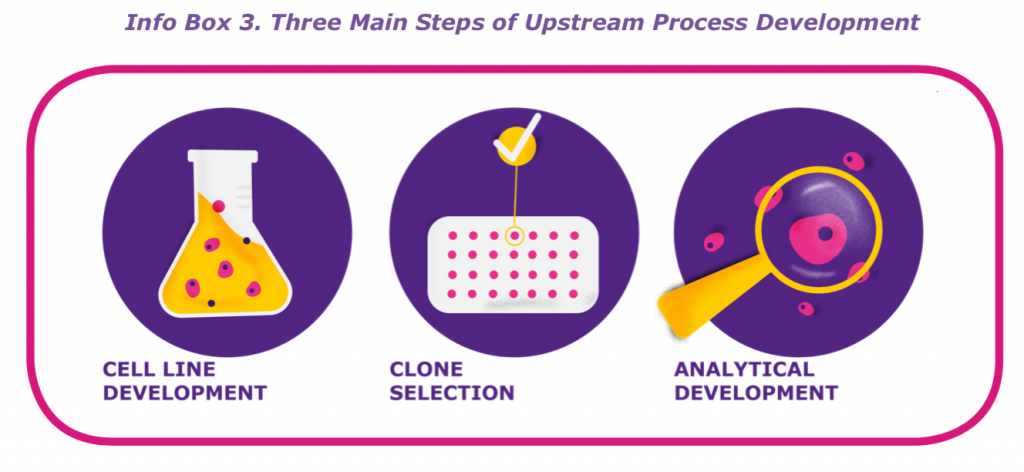
One of the first stages of biological drug development is upstream process development, which consists of the three stages cell line development, clone selection, and analytical development. The key purpose of upstream process development is to develop and grow stable cell lines in bioreactors that can then produce biomolecules, such as antibodies and recombinant proteins.
 When upstream process development is completed, the drug enters the manufacturing stage. Here, early-stage biotechs are confronted with new challenges in biological drug development, such as ensuring that upscaling solutions are in place to meet patient demand and avoiding contamination of the product, which might pose a risk to patients in the clinic.
When upstream process development is completed, the drug enters the manufacturing stage. Here, early-stage biotechs are confronted with new challenges in biological drug development, such as ensuring that upscaling solutions are in place to meet patient demand and avoiding contamination of the product, which might pose a risk to patients in the clinic.
Once the biotech has ensured the safety and pharmacological activity of its biological compound, it can file for IND (investigational new drug). Now, the biologic is subjected to new requirements within the regulatory system. A suitable and experienced drug development partner can be of great help in these situations.

Working with the regulatory authorities early on can ensure streamlined processes and mitigate risks. It is always important to remember that the key purpose of the regulatory authorities is to ensure patient safety in biologics drug development. Early-stage biotechs should therefore have sufficient data to show that their biologic will not jeopardize patients’ health and safety in phase I.
Although early-stage biotechs face a number of challenges throughout biological drug development, there are quite a few things they can do to mitigate the risks. By building a robust strategy, finding experienced partners, and continuously assessing risks, biotechs can successfully progress through biological drug development.
Images by E. Resko






