News and Trends 31 Aug 2022
Emactuzumab designated as an orphan medicinal product for TGCT in Europe
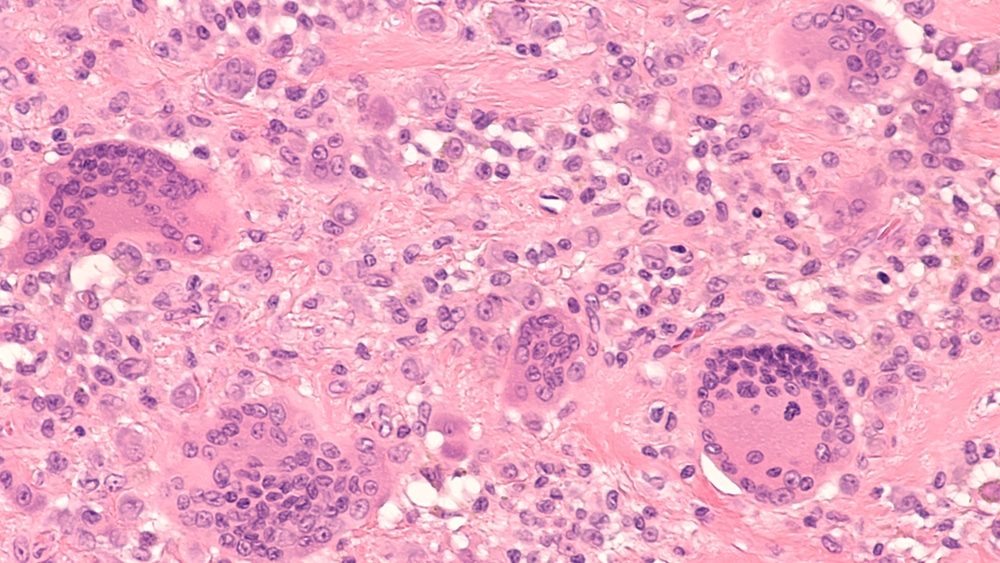
SynOx Therapeutics Limited says its novel monoclonal antibody, emactuzumab, in development for the treatment of tenosynovial giant cell tumor (TGCT) and other diseases, has been designated as an orphan medicinal product for both localized and diffuse types of the disease by the European Medicines Agency (EMA). No systemic treatments have been approved for TGCT in […]