Newsletter Signup - Under Article / In Page
"*" indicates required fields
Blockbuster drugs are raking in billions each year in sales. Let’s have a look at the top 7 European best-seller drugs of 2016 and what their competition has to say.
Not surprisingly, the top 3 best-selling drugs in Europe are biologicals, which are revolutionizing the treatment possibilities for a range of conditions, from cancer to chronic diseases. But with the increasing number of biosimilars as well as generics on the way, it seems that for many of these candidates competition is working hard on keeping up.
Here we had a closer look at the blockbuster drugs of 2016, what diseases they treat and who else is in the game waiting for their patents to expire.
2016 sales: €4.089B (£3.485B), – 5.3% since 2015
GlaxoSmithKline‘s Advair is used in the management of asthma and chronic obstructive pulmonary disease (COPD). Advair contains an inhalable combination of the anti-inflammatory corticosteroid Fluticasone as well as the long-acting beta-adrenoceptor agonist Salmeterol, which treats constriction of the airways.
The European patent for this combination expired in 2013, and the generic company Mylan is currently awaiting FDA approval for its Advair generic.
2016 sales: €4.769B ($5.045B) (€1.625B Bayer + €3.144B Regeneron), + 26.9% since 2015
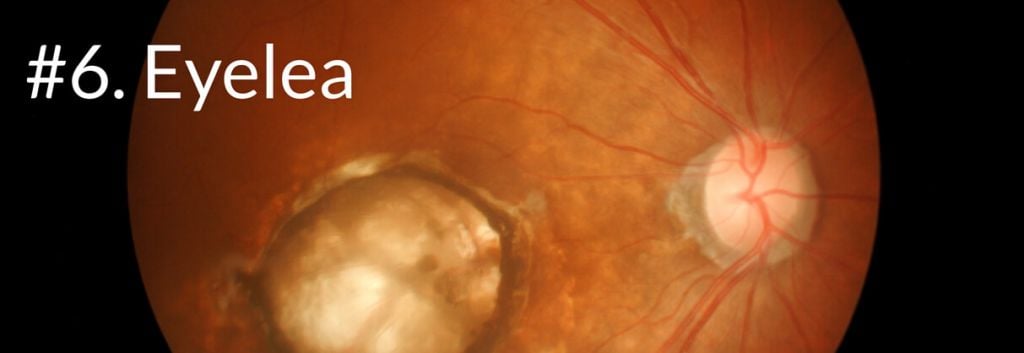
Eyelea (Afllibercept) was developed by the US biotech Regeneron, which partnered up with Bayer to develop the drug for the treatment of wet macular degeneration in 2006. Aflibercept is a recombinant fusion protein consisting of vascular endothelial growth factor (VEGF)-binding portions, that are fused to the Fc portion of the human IgG1 immunoglobulin.
The fusion proteins bind VEGFs thereby inhibiting their activity and thus growth of blood vessels within the eye. Such leaky, abnormal blood vessels are characteristic for this disease.
2016 sales: €5.097B ($5.390B) (€2.928B Bayer + €2.169B J&J), + 26.7% since 2015
Xarelto (Rivaroxaban) is a blood thinner, which was originally developed by Bayer and is marketed by J&J in the US. It is a highly specific factor Xa inhibitor, a component of the blood coagulation cascade and it is the first available active factor Xa inhibitor which is taken orally. The target indications are venous and arterial thromboembolic conditions.
2016 sales: €5.714B ($6.054B), – 10.6% since 2015
Insulin glargine, marketed under the name Lantus, is Sanofi‘s long-acting insulin analogue. given once daily to help control the blood sugar level of those with diabetes. It consists of microcrystals that slowly release insulin, giving a long duration of action of 18 to 26 hours, with a “peakless” profile.
In 2015, Sanofi also launched the first US-approved inhaled insulin and recently received FDA approval for a Lantus combination therapy. Lantus is also being chased down by biosimilars probably causing the loss in sales compared to 2015. Its patent expired in 2015 and the Lantus biosimilar by Eli Lilly and Boehringer Ingelheim was approved in 2014.
2016 sales: €6.328 billion (CHF 6.782 billion), + 3.7% since 2015
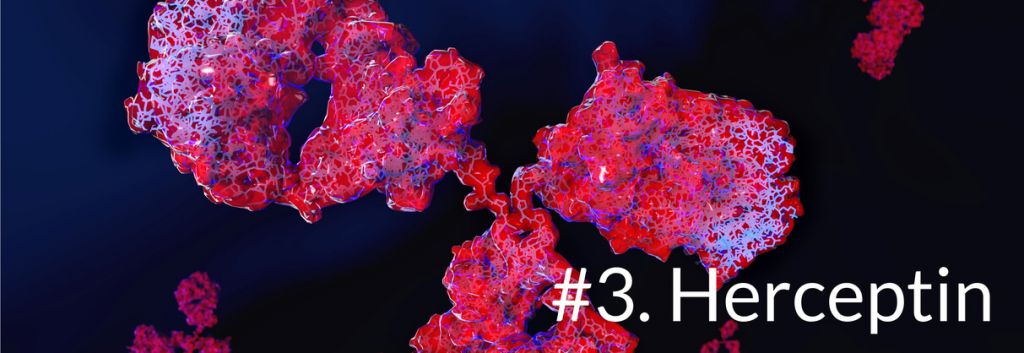
Herceptin (trastuzumab) from Roche is a is a monoclonal antibody used to treat breast cancers, which are HER2–positive. The antibody binds to the HER2 receptor overexpressed on some breast cancers, thereby inhibiting the proliferation of these cells and marking them for antibody-mediated clearance by the immune system.
Its patent went off in 2014 in Europe (when Biocon and Mylan got a biosimilar approved), but Roche still has the US until 2019. Together with Avastin and Rituxan, these 3 drugs made Roche the leader in cancer treatments.
 2016 sales: €6.327B (CHF 6.783B), + 1.5% since 2015
2016 sales: €6.327B (CHF 6.783B), + 1.5% since 2015
The 2nd place also filled by one of Roche‘s anti-cancer biologicals Avastin (bevacizumab). This antibody inhibits the vascular endothelial growth factor A (VEGF-A) and thereby blocks angiogenesis, one of the hallmarks of cancer. Roche’s patent in the US expires in 2019, whereas it still has its patent in Europe until 2022. Still, Amgen and Allergan are already working on developing a biosimilar for this cancer therapy.
2016 sales: €8.109B ($8.583B) (€6.810B Roche + €1.299B Biogen), + 2.7% since 2015
And the first place goes to… Roche once more! Rituxan (rituximab) is another monoclonal antibody, which targets the CD20 protein found on the surface of B cells. The antibody is used for the treatment of B-cell leukemias and lymphomas as well as B cell-associated autoimmune diseases such as Rheumatoid Arthritis.
Roche’s patent expired in 2015 and now faces biosimilar competition. Truxima was recently approved as the world’s first biosimilar with an oncology indication. But the market is crowded: Novartis’ daughter company Sandoz applied for European marketing authorization for its rituximab biosimilar in May 2016 and both Pfizer and Amgen have their biosimilars in development.
Images via shutterstock.com / 279photo Studio / memorisz / Victor Josan / Jarva Jar / Juan Gaertner / Anna Jurkovska / vitstudio / Pushish Images
Artificial intelligence and the future of antibody discovery