Ablynx has shown that its candidate for Rheumatoid arthritis could work as a monotherapy, but whether they can convince AbbVie to stick to the previous $840M deal is still unclear.
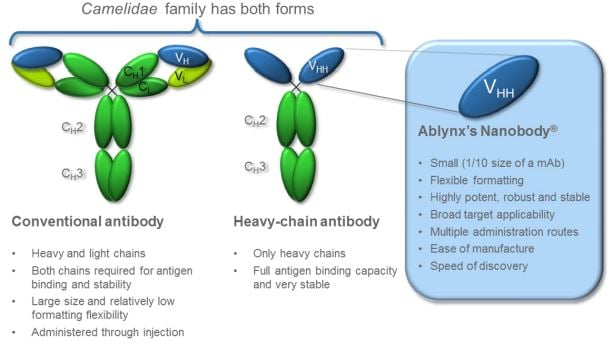
 Ghent-based Ablynx has hooked several big Pharmas with its Nanobody platform, based on the shorter monoclonal antibodies of llamas.
Ghent-based Ablynx has hooked several big Pharmas with its Nanobody platform, based on the shorter monoclonal antibodies of llamas.
One of these is AbbVie (France), which is trying to get a new rheumatoid arthritis candidate to replace its super-successful blockbuster Humira, now under biosimilar attack.
Back in 2013, it paid Ablynx $175M upfront for a collaboration to develop vobarilizumab, an anti-IL-6R antibody. The candidate targets a receptor for interleukin-6, a protein involved in inflammation. Interleukin-6 is also the target for the rheumatoid arthritis candidates of Janssen’s sirukumab and Sanofi’s sarilumab.
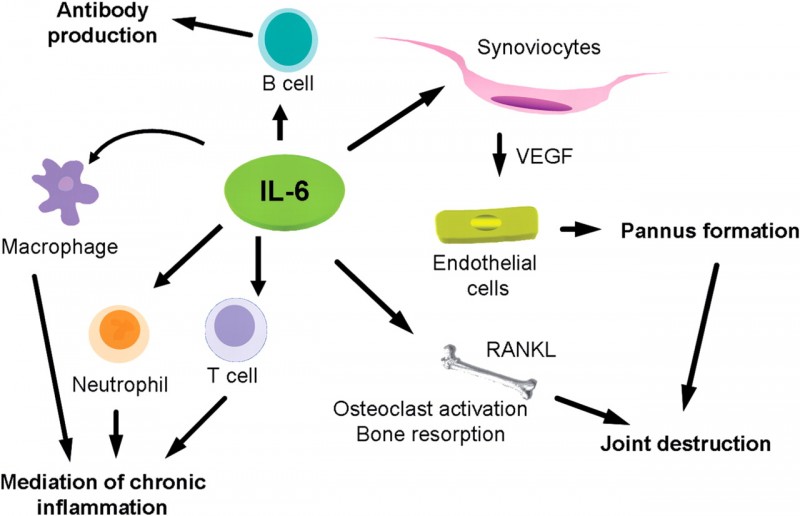
Now, results for vobarilizumab’s Phase IIb trial as a monotherapy for rheumatoid arthritis are out. The trial enrolled 251 patients with rheumatoid arthritis that are intolerant to methotrexate (common drug for psoriasis and rheumatoid arthritis) – hence the interest in a monotherapy.
As it is, Ablynx’s candidate appears to work. While measuring the improvement in rheumatoid arthritis is somewhat technical, in rough terms 73% to 81% of patients have seen at least 20% of improvement in symptoms (ACR20). There was also a 41% rate of remission, which Ablynx deems “very encouraging”.
However, the problem with the results may not be if it works, but rather how well it works in comparison with its main competitor. That is Roche’s Actemra (tocilizumab), also an anti-IL-6R therapy.
Ablynx’s trial included a group of patients knowingly being treated with Actemra (open-label). In this group, 78% of participants met ACR20. Not so much of a difference between vobarilizumab’s effect…
Therefore, AbbVie may think twice before picking up vobarilizumab for further development. If it does, it will be giving €68M ($75M) to Ablynx as a milestone payment, as well as financing the ensuing Phase III trial and commiting to further milestones and royalties – which could go up to €385M ($425M).
There are other criteria that could give Ablynx’s candidate an edge, however. It seems to have a better safety profile and has to be taken less regularly – every two weeks instead of every week.
Even so, AbbVie has famously let go of Galapagos’ candidate for rheumatoid arthritis despite good results and when a opt-in looked like a sure thing.
Feature Image Credit: Pixabay
Figure 1 Credit: Dayer and Choy (2010) Therapeutic targets in rheumatoid arthritis: the interleukin-6 receptor, Rheumatology (doi: 10.1093/rheumatology/kep329)





