Newsletter Signup - Under Article / In Page
"*" indicates required fields
Arcutis Biotherapeutics, Inc. has entered into an agreement to acquire Ducentis BioTherapeutics Ltd., a privately held, preclinical-stage biotechnology company focused on developing novel therapies for inflammation and autoimmune diseases.
Arcutis BioTherapeutics will acquire the outstanding shares of Ducentis for an upfront cash payment of approximately $16 million and Arcutis stock valued at approximately $14 million, as well as future contingent payments based on development and commercial success. Closing of the transaction will be subject to customary closing conditions.
Ducentis’ lead asset is DS-234, a fusion protein that is a highly selective and potent agonist of the CD200 receptor (CD200R). CD200R is an immune-regulatory receptor that is thought to be an important immunological checkpoint with a pivotal role in the maintenance of immune tolerance.
Checkpoint agonism is an emerging immunomodulatory approach that works to amplify pathways that inhibit overactive immune cells and suppress unwanted immune responses. DS-234 binds to CD200R, restoring immune homeostasis by inducing inhibitory signaling on immune cells that regulate inflammation.
CD200R has been validated as a target in atopic dermatitis, with recent preclinical and clinical data providing evidence of a durable biologic response, even after discontinuation of treatment. Ducentis has completed preclinical comparisons of DS-234 against the clinically-validated CD200R antibody. The data compare favorably across key metrics including potency, efficacy, and pharmacokinetics, offering potential differentiation through an improved ability to modulate the CD200 pathway, a longer half-life, and a higher steady state volume of distribution.
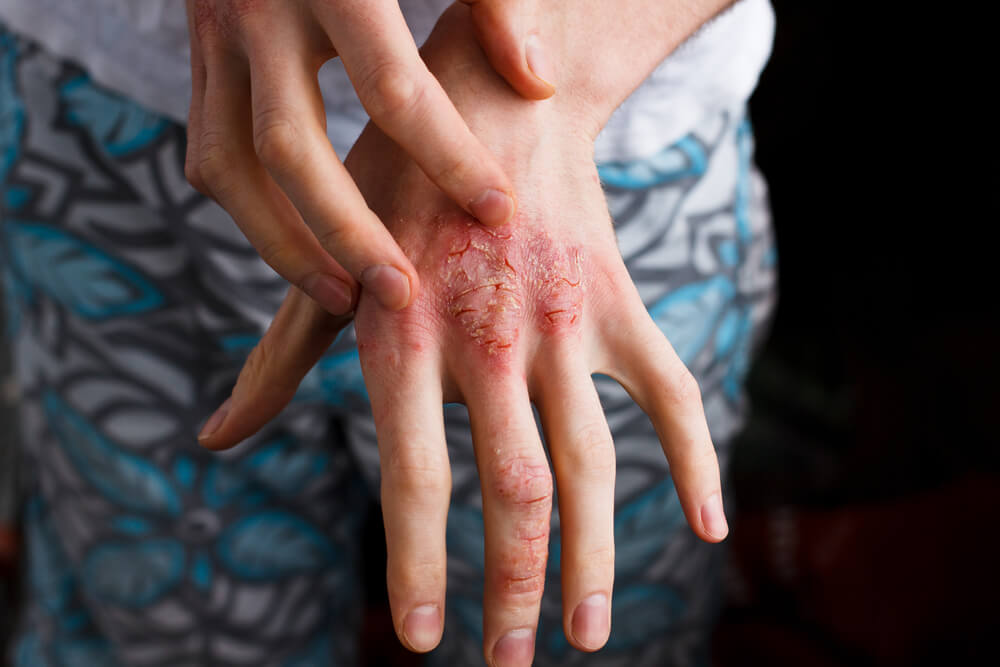
About atopic dermatitis
Atopic dermatitis is the most common type of eczema, affecting approximately 9.6 million children and 16.5 million adults in the U.S. alone. It is characterized by a defect in the skin barrier, which allows allergens and other irritants to enter the skin, leading to an immune reaction and inflammation. This reaction produces a red, itchy rash, most frequently occurring on the face, arms, and legs. The rash can cover significant areas of the body, in some cases impacting half of the body or more.
Strategic acquisition for Arcutis Biotherapeutics
“This acquisition is an important step in realizing our vision of becoming the leading, innovation-driven medical dermatology company, and leverages our deep dermatology expertise and capabilities across the organization to develop, manufacture, and commercialize therapies that address important unmet needs across the continuum of care,” said Frank Watanabe, Arcutis BioTherapeutics president and chief executive officer.
“Ducentis’ DS-234 fits in well with our strategy of developing potential best-in-class molecules against biologically-validated targets and is highly complementary to roflumilast cream as another potential innovative treatment option. We are excited by the promise of checkpoint agonism as an emerging strategy for the treatment of atopic dermatitis. Additionally, with the majority of our clinical, manufacturing, and commercial teams already possessing experience with biologic agents, DS-234 fits well with our team’s expertise. With a modest investment, we believe we can generate proof-of-concept data against a de-risked target in a high-value indication.”
“Ducentis is thrilled to join Arcutis, which has the resources, experience, and commitment needed to accelerate the clinical development of DS-234 as an important new treatment option for patients with atopic dermatitis, and in the future, other serious autoimmune diseases lacking effective treatment options,” said Philip Huxley, founder and former chief executive officer of Ducentis.
“With Arcutis’ depth of knowledge and capabilities in dermatology, and its team’s experience developing, manufacturing, and commercializing biologics, we are confident that Arcutis is well positioned to build on the pre-clinical work that the Ducentis team has completed to date.”
Ducentis’ chief scientific officer, Rebecca Ashfield, will be retained by Arcutis BioTherapeutics as a consultant to ensure knowledge transfer, integration of ongoing workstreams, and lead future technical and manufacturing operations related to DS-234.
Partnering 2030: Biopharma Report