Newsletter Signup - Under Article / In Page
"*" indicates required fields
Certa Therapeutics has announced what it says are ground-breaking clinical trial data for FT011, its novel therapy for the treatment of serious inflammatory and fibrotic diseases.
Certa Therapeutics has recently completed a global phase 2, multi-centre, randomized, double blind, placebo-controlled study of the pharmacokinetics, pharmacodynamic effects, and safety, of oral FT011 in patients with scleroderma.
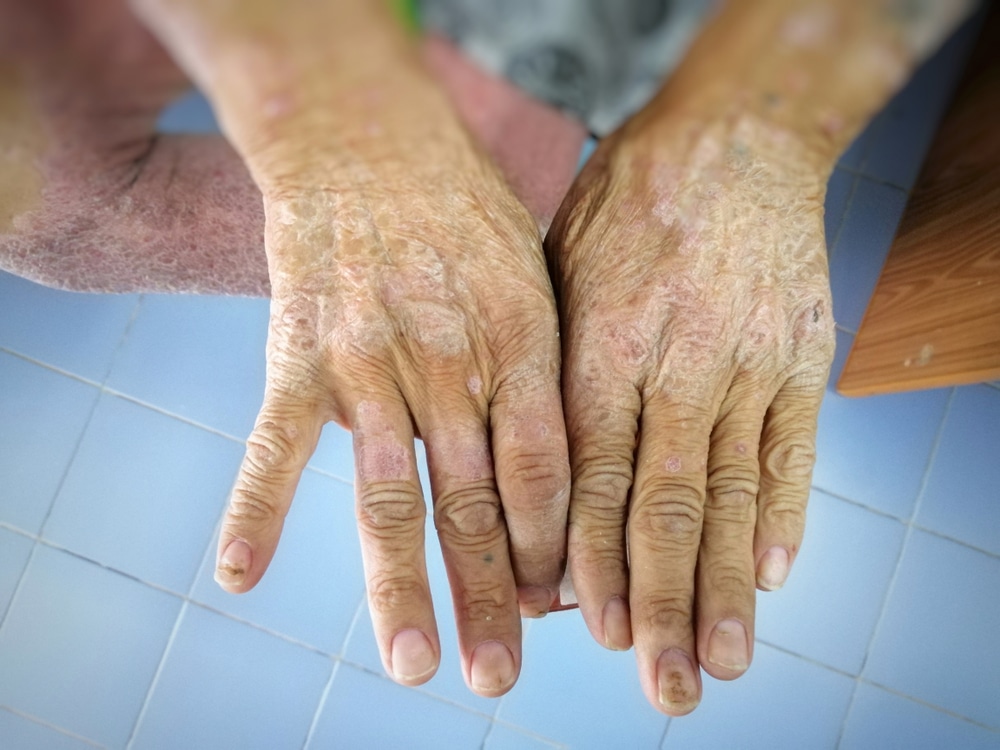
Scleroderma is a debilitating, potentially life-threatening autoimmune condition characterized by inflammation and fibrosis of the skin and other organs (commonly the lungs, kidneys, and heart). This condition results in high morbidity with substantial detriment on quality of life, with patients commonly experiencing loss of mobility and function, pain, fatigue, often accompanied with a significant impact to their mental health. Scleroderma has the highest mortality among rheumatic diseases.
Certa Therapeutics‘ study results
The study results show that, compared to placebo, treatment with FT011 led to significant improvements across multiple efficacy measures including; the American College of Rheumatology Combined Response Index in diffuse cutaneous Systemic Sclerosis (CRISS) score, skin thickness (defined by the modified Rodnan Skin Score, mRSS), lung function (%FVC), physician-reported assessment and quality of life evaluations. The ARC-CRISS results describe a composite measure of disease state and organ damage that predicts the probability of improvement from baseline in scleroderma patients as a range between 0.0 to 1.0, with a score of greater than 0.6 indicating a clinically meaningful improvement.
Certa Therapeutics’ phase 2 trial demonstrated that treatment with FT011 resulted in a clinically meaningful improvement in 60% of patients treated with FT011 400mg and 20% of patients in the FT011 200mg group.
Three patients in the pooled FT011 group achieved a maximum CRISS score of 1.0, representing the greatest probability of clinical improvement.
Certa Therapeutics CEO and founder Darren Kelly said: “These exceptional trial results demonstrate the potential of this novel treatment for patients with scleroderma. The changes seen in CRISS score, lung function, and physician reported outcomes in addition to the patient reported outcomes within such a short treatment timeframe of 12 weeks, is unprecedented and paves the way for a confirmatory global phase III study.
“We know that this debilitating and life-threatening disease can severely impact the lives of patients and to date existing treatments only focus on the relief and management of symptoms, whereas FT011 precisely targets the root cause of fibrosis and has the potential to offer treatment across multiple organs within these patients.”
Extension ongoing
The trial recruited 30 adults who were randomly assigned to three treatment arms: FT011 400mg or FT011 200mg or placebo daily, in addition to standard of care, for 12 weeks.
When compared to placebo, both FT011 treatment groups showed clinically meaningful improvements in CRISS score as early as week 8 and reaching a maximum at week 12.
An open label extension phase of the trial is ongoing where a subset of patients who completed the main study (up to week 12), have elected to remain on treatment (FT011 400 mg) for an additional 9 months.
In addition to the outstanding CRISS score results, the breadth of clinical benefit of FT011 in scleroderma patients was also indicated through the change in mRSS, which showed notable changes in skin thickness for patients treated with FT011. Five patients in the pooled FT011 group recorded substantial changes in mRSS of -8 or greater at week 12.
For patient reported outcomes assessed using the Scleroderma Health Assessment Questionnaire Disability Index (SHAQ-DI) 3 over the 12 weeks, the 400 mg FT011 group showed a statistically significant improvement while the placebo group demonstrated a worsening in outcomes. Of the health assessment components that contribute to the SHAQ-DI, the FT011 400 mg group demonstrated statistically significant improvements over 12 weeks of treatment when compared to placebo for pain, breathing problems, finger ulcers and overall disease activity.
No adverse events
Global physician assessments of the patients’ health status showed a significant difference in favor of the 400 mg FT011 group vs. placebo at 4, 8 and 12 weeks.
The study safety profile demonstrated that FT011 was safe and well tolerated, with no differences in adverse event (AE) rates between the treatment arms. There were no serious AEs reported in the study, nor any AEs resulting in study drug interruption, withdrawal, or discontinuation.
Wendy Stevens, a rheumatologist at St Vincent’s Hospital Melbourne, and a principal investigator in the FT011 trial, said: “The trial demonstrated that FT011 was safe and well tolerated. Scleroderma can lead to severe and life-threatening issues and patients with the condition often face daily struggles as a result of their symptoms. These results are a significant step towards helping patients with this debilitating disease. Further longer and larger trials of this medication are now needed to assess its potential to improve this debilitating condition.”
Certa Therapeutics’ phase 2 results add to the extensive body of data regarding its lead candidate FT011.
FT011 has demonstrated promising efficacy in multiple non-clinical efficacy models of fibrotic disease, with recent transcriptomic research demonstrating that treatment with FT011 results in reversal in the activation of genetic markers associated with fibrosis, 4 as well as an excellent safety profile in phase 1 studies.
Certa Therapeutics is developing other novel therapies focused on treating fibrotic diseases which account for 45% of all deaths globally in the industrialized world, demonstrating an unmet need.
Certa Therapeutics’ global phase II trial in scleroderma patients provides a promising indication of FT011’s potential efficacy in treating inflammation and fibrosis more generally as scleroderma affects most organs in the body.