Newsletter Signup - Under Article / In Page
"*" indicates required fields
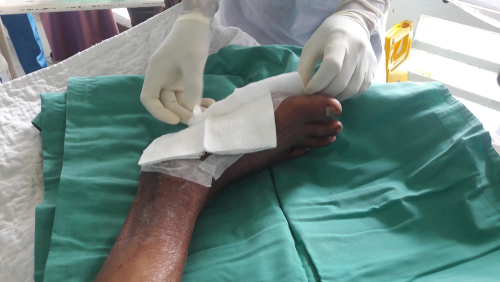
Cambridge U.K. biotech company SolasCure, which is developing a wound cleaning technology, has published its pre-clinical results with the International Wound Journal, showing preliminary evidence that supports its new enzymatic debridement product for use in chronic wounds.
Debridement, the removal of all infected and nonviable tissue, is a crucial prerequisite for a wound to heal.
Leveraging biomimicry and evidence-based medicine, SolasCure is developing Aurase Wound Gel, a hydrogel containing an enzyme cloned from medical maggots that can be used to accelerate wound cleaning and support healthcare professionals treating patients with chronic wounds.
The pre-clinical development, which included in-vitro and in-vivo applications of the Aurase Wound Gel, has produced data that supports the potential for this maggot-derived proteolytic enzyme as a clinically effective debriding agent.
Good safety profile for SolasCure technology
The enzyme is shown to have a good safety profile both locally and systemically, providing for a large therapeutic window, which has permitted SolasCure under regulatory review to test the enzyme on real patients with ulcers.
Sam Bakri, founder and CEO of SolasCure, said: “Chronic wounds pose a substantial and growing clinical, social, and economic challenge, with increasing healthcare costs, an aging population, and the continued threat of diabetes and obesity across the globe.
“As SolasCure is motivated by the potential for disruptive new, life-changing technologies, we are delighted to now share these encouraging results with the wound care community. They are a significant milestone for SolasCure and its partners, as is the recognition from the IWJ of the need for an effective enzymatic debrider and the potential of Aurase.”
Publication of the pre-clinical results in the IWJ comes as the final patient has been enrolled in SolasCure’s clinical trial, which began in 2021 and includes centers across the U.K., U.S., and Hungary.
Results from the clinical trial are expected this year.