Lyon’s Erytech (France) is a clinical biopharma working on improving its red blood cell transported drug, targeting cancers such as acute lymphocytic leukemia (ALL) and pancreatic adenocarcinoma. Now phase I/II trials for its GRASPA therapy have demonstrated good efficacy and safety.
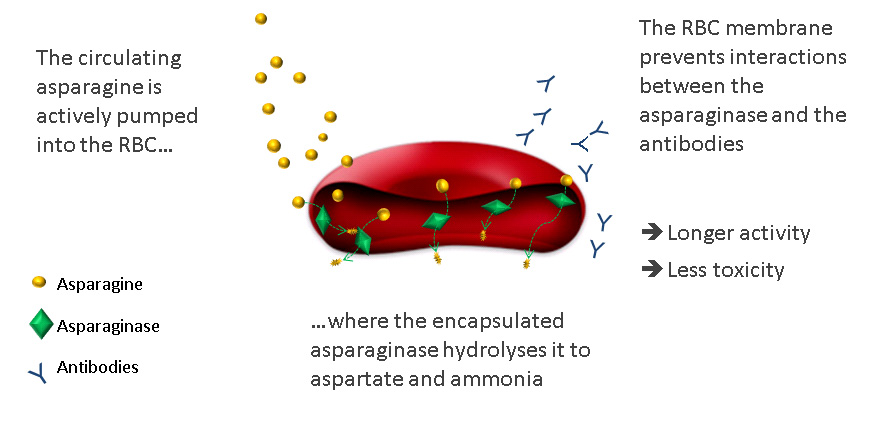
 Circulating asparagine is needed by cancer cells to grow and proliferate. Erytech’s platform uses a different approach – insertion of an oncology drug (the enzyme L-asparaginase) in red blood cells, which circulates and delivers the drug, and therefore reduces serum asparagine. This starves cancer cells and induces them to undergo apoptosis.
Circulating asparagine is needed by cancer cells to grow and proliferate. Erytech’s platform uses a different approach – insertion of an oncology drug (the enzyme L-asparaginase) in red blood cells, which circulates and delivers the drug, and therefore reduces serum asparagine. This starves cancer cells and induces them to undergo apoptosis.
Erytech has applied for EU market approval for their ERY-ASP (‘Graspa’) drug to treat ALL in up to 38 countries. Now, Graspa is also undergoing phase II trials for elderly patients (i.e. >55 years old) with a similar type of leukemia, as well as a phase II study for its treatment of metastatic pancreatic adinocarcinoma. Both phase I trials showed excellent tolerability of the drug.
So, having conquered the EU market for ALL, Erytech is now also well on its mission to jump the pond into the US leukemia market, with their ongoing phase I trial over there. Since over 50,000 people are diagnosed with ALL or AML across the US and EU each year, it is understandably a desirable market to segway in on. However, the FDA & EMA has granted orphan drug designation for Graspa in all 3 cancer types…instead making Erytech the object of interest.
Erytech currently has a market cap of €201.6M, and has announced plans for an additional IPO launch on NASDAQ in the US this July. Their first IPO back in 2013 was rather successful, raising a total of €17.7M and attracting partnerships with Israeli TEVA and Oprhan Europe. On a side note, there generally appears to be a significant amount of interest in therapies for ALL in Israel (e.g. Gamida Cell) which we recently wrote about, so it was interesting to hear about TEVA’s marketing approval for Gaspa.
Erytech’s platform is certainly different from other oncology drug delivery designs out there, and we suspect there will be even more investor interest in this French biotech as Graspa’s progress continues.