Molecular Health, a bioinformatics company based in Heidelberg, Germany, today announced that it will enter a commercial-licensing agreement with the US Food and Drugs Standard Agency (FDA) to implement their new bioanalytical product SafetyMAP to analyse risk of marketed drugs.
Molecular Health (Labiotech Map Profile here), partnered with MD Anderson (TX) early on, doing consultancy for the cancer research giant before the launching of their bioinformatics research could go ahead. This permitted Molecular Health the time needed to deepen their understanding of the clinical data system and adapt their approach to data-mining within the medical research field.
By data mining current journal publications and collating it with molecular data from patients, MH developed their ‘Nucleus’ technology which homogenises these disparate sources of information in order to create comprehensive analyses of particular drugs and their trials, down to the target-pathway level.
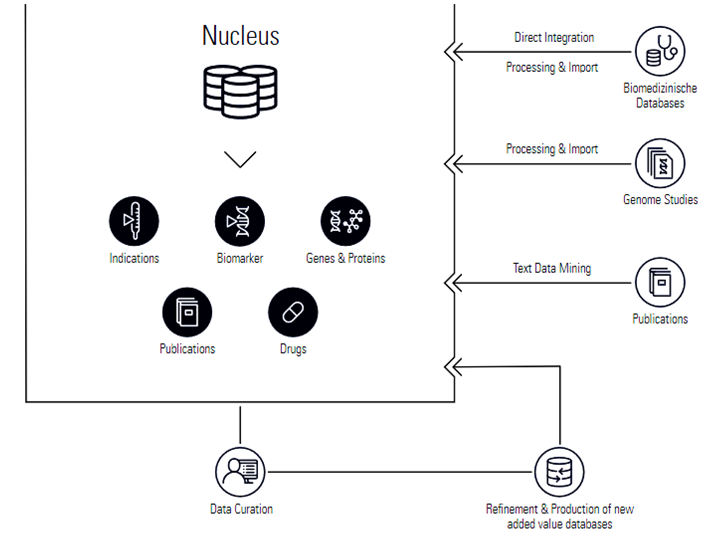
From here, Molecular Health managed to engineer the first of their key data-mining platforms – Molecular Analysis of Side Effect Information (MASE). MASE functions by mining various different sources of medical data such as MEDLINE, FAERS, gene and protein databases, FDA labels for licensed drugs, patents, and other publications. In doing so, MASE is able to form calculated risks regarding particular drugs, treatments and clinical trials which can be analysed from the perspective of certain molecular parameters.
This all seems rather impressive, particularly when viewed from a non-bioinformatics level. However, data-mining is becoming increasingly common, with advanced programming permitting products such as SafetyMap to be pitched out of MASE research to federal agencies as big as the US Food and Drugs Standard Agency (FDA) – which have never before used this kind of bioinformatic technology.
In 2012, a research agreement was reached with the FDA to streamline its MASE application in the form of calculating risk and side effects of drugs; thus SafetyMap was born. Pitched as a ‘data healthcare analytics tool for the molecular analysis of drug induced side effects’, SafetyMap will be licensed to analyse all marketed-drugs for up to 1 year within the FDA, with rights extending to 10 concurrent users.
Other MASE spin-off projects include TreatmentMAP – a molecular profiling and treatment service provider using Next Generation Sequencing (NGS) in order to profile a patients tumor from DNA samples. This is then used by medical professionals to consult and weigh-up preferential treatments regarding target risk and potential drug resistance.
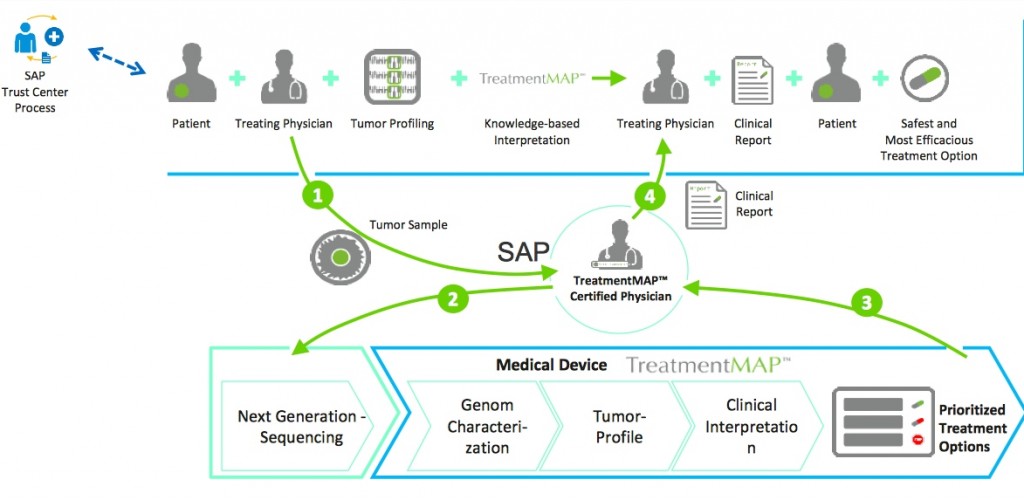
Molecular Health is also planning to pitch InsightMAP to pharmaceutical biotechs as a customised version of TreatmentMAP building on its Nucleus technology. MH is hoping InsightMAP will be used alongside clinical trials and aid in the discovery of new biomarkers, drug re-purposing and running companion diagnostics.
Their work is becoming increasingly essential to biotechs in cancer research, with the overwhelming volume of clinical and molecular data circulating in the global academic field. It is therefore not surprising to see the FDA enter into a license agreement, evidently appreciating the value of data-mining as the way forward in drug-testing and pharmaceuticals.
Bonus from our previous documentary in South Germany, an interview of Molecular Health’ CEO: