That’s what the China Food and Drug Administration (CFDA) declared on the blood diagnostic test from Berlin-based Epigenomics‘ proprietary Septin9-Biomarker. Their Septin9 test Epi proColon is filling the need for early detection of colorectal cancer. Data show a huge increase in prevalence in urban China. Just last week we interviewed the CEO of Epigenomics, Thomas Taapken, on the company’s Cancer detection technology and Septin9 test Epi proColon. The colorectal cancer screening test is the first of its kind and has been approved by the EU, CFDA and now by the FDA in the US, too.
Just last week we interviewed the CEO of Epigenomics, Thomas Taapken, on the company’s Cancer detection technology and Septin9 test Epi proColon. The colorectal cancer screening test is the first of its kind and has been approved by the EU, CFDA and now by the FDA in the US, too.
Imagine, a simple blood test which can detect one of the deadliest types of cancer.
Indeed over 290 million people in China are estimated to be eligible to colorectal cancer screening, and early detection is the key to saving lives. The Chinese government has even highlighted early CRC screening as a focus in Cancer prevention.

And this type of cancer is on the rise…with one report estimating that the number of colorectal cancer cases between 2012 and 2022 in urban areas of China will double.
This is a shocking statistic, so an early-diagnostic system is therefore a really big deal – and getting named one of the most ‘innovative medical products’ from the CFDA is a further validation of this test! According to Taapken:
This is excellent news and recognises the potentially huge contribution we can make to early colorectal cancer detection in China with our proprietary biomarker Septin9.“
Approval from the CFDA is not an easy achievement either! According to the recently published ‘2015 Medical Device Registration Annual Report’, only 9 out of 7530 approved medical devices received this label by the Chinese regulators, who acknowledged the significant clinical value of the products for the population.
BioChain Institute and its affiliate BioChain (Beijing) Science and Technology Corp. worked with Epigenomics to bring the Septin9 test to the Chinese market. The relationship between the two partners is sure to grow into an even more ‘fruitful collaboration’, as both celebrate this breakthrough.
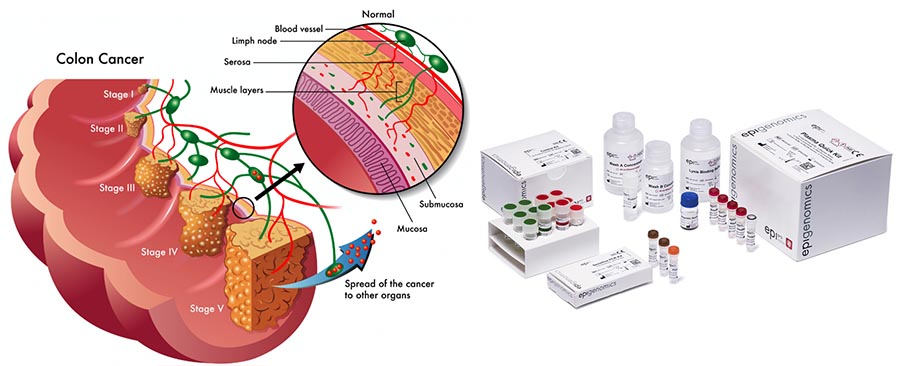
So how does the Septin9 test work?
Based on its DNA methylation technology, Epigenomics has developed a platform called Epi proColon which can detect minute concentrations of colorectal cancer specific DNA fragments (shed into the blood stream by tumor cells).
As we discussed, the advantages of such a test are numerous. For example, in comparison to existing methods it is more convenient and easier to use for health care professionals and patients.
And the preference of a minimally invasive blood test over a colonoscopy is understandable, as surgical procedures can require over 24 hours preparation (which is a very unpleasant experience for the patient).
With Epi proLung, Epigenomics is developing a second blood-based test for the detection of lung cancer – the most deadly type of cancer. In Time Magazine a recent report estimates the incidence of lung cancer to reach up to 800,000 new patients each year in China by 2020.
So as of last month, Epigenomics and BioChain extended their strategic licensing agreement to also include such a blood test for lung cancer too.
This could be a real breakthrough in the detection of cancer patients in China.





