Epistem’s molecular diagnostic (Genedrive) has achieved 100% accuracy in detecting a genotype relevant to Hepatitis – an important proof of concept for this field device.
![]() Located in Manchester (UK), Epistem has a market cap of €130,000 and 29 employees.
Located in Manchester (UK), Epistem has a market cap of €130,000 and 29 employees.
One of its research areas is molecular diagnostics, under which it developed Genedrive. This device can make test for genotypes (gene variants) in under 60 minutes, enabling low cost and high sensitivity diagnostics at the point of care – such as clinics.
Now Epistem has announced that one of its available assays (for the Hepatitis C virus) has been validated in an independent clinical trial conducted by the Institut Pasteur and Hôpital Cochin (Paris).
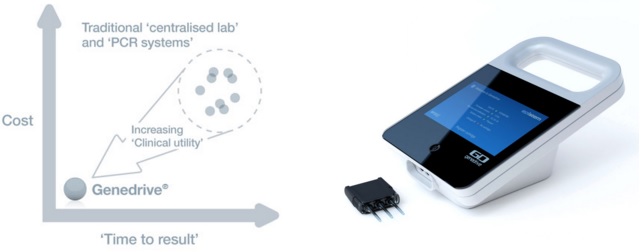
The assay only requires a mouth swab, and can distinguish between different genotypes of the IL28B gene – which codes for a cell-signalling protein (cytokine) involved in the immune response to viral infection.
Certain variants (single nucleoside polymorphisms – or SNPs) of this gene are associated with better responses to certain drugs, in the treatment of hepatitis C virus (HCV). Given this, knowing the patient’s genotype was important to prescribe the right treatment.
The clinical relevance of these HCV diagnostics has waned since pan-genotypic oral drugs became available. These are treatments which act on all the different genotypes of HCV – and therefore can treat a very large percentage of the infected population. For example, Gilead’s Sovaldi (sofosbuvir).
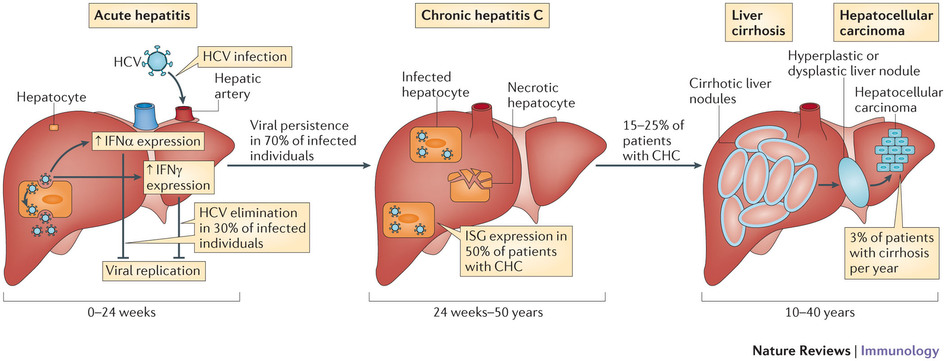
However, the IL28B assay may still be of interest to less wealthy countries, as Sovaldi’s steep price is a challenge to public health systems.
These results also open doors to other type of tests, including detection of RNA from HCV – which could rapidly screen for infection.
Besides HCV, Genedrive also has developed assays to test variants of the OPRM1 gene (connected with a predisposition to addiction), tuberculosis infection, and even for pathogens used in bioterrorism.
Genetic diagnostics are increasingly making it into the market – be it in rare diseases or cancer. What will be the future of Epistem’s strategy of handheld tests?