Newsletter Signup - Under Article / In Page
"*" indicates required fields
The collaboration between AstraZeneca and the money-magnet Moderna is giving its first steps. AstraZeneca is filing for authorization of a German Phase I trial, to assess an mRNA therapy for cardiometabolic diseases.
![]() Therapies using mRNA have a lot of potential as biologics. Leading Biotechs in the field include CureVac (interview with its CCO here) and BioNTech, which recently published exciting results.
Therapies using mRNA have a lot of potential as biologics. Leading Biotechs in the field include CureVac (interview with its CCO here) and BioNTech, which recently published exciting results.
On the other side of the Atlantic, the also mRNA-focused Moderna Therapeutics has been very successful, as we discussed with CEO Bancel. It raised over $900M dollars in VC money. A key investor is Flagship Ventures, which has attracted AstraZeneca and other industry players.
In January, AstraZeneca struck a deal with Moderna to co-develop mRNA candidates in the clinical stage. The scope of the agreement included treatments for cardiovascular, metabolic and renal diseases as well as cancer.
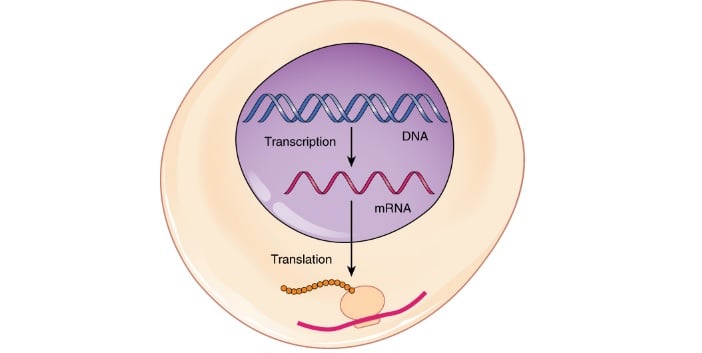
Now, this agreement has borne its first fruit. AstraZeneca is moving to get authorization for a Phase I trial, having filed a Clinical Trial Application (CTA) with regulatory bodies in Germany (Paul Ehrlich Institute and the German Federal Ministry of Health).
The trial will evaluate AZD8601, an investigational mRNA-based therapy that encodes for vascular endothelial growth factor-A (VEGF-A). This protein stimulates the formation of blood vessels and is more famous for its role in cancer. Certain cancer drugs inhibit VEGF, such as Roche‘s Avastin.
This therapy for cardiometabolic diseases does the opposite, instead raising the levels of growth factor. The objective is to initiate a strong, local and transient surge of VEGF-A expression.
The program is built upon research on heart stem cells, which found that VEGF-A can act as a cell fate switch for cardiac progenitors – directing stem cells to make necessary cardiovascular cells.
AstraZeneca believes this therapy could one day provide a unique regenerative treatment option for patients with heart failure, diabetic wound healing and other ischemic vascular diseases.
The company has shown interest in the field lately, having for example signed a €100M deal for a for lipid disorders candidate with the Swedish Lipigon Pharmaceuticals.
Feature Credit Image: AstraZeneca
Figure 1 Credit: Adams and Alitalo (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nature Reviews Molecular Cell Biology (doi: 10.1038/nrm2183)






