Newsletter Signup - Under Article / In Page
"*" indicates required fields
Ablynx, the llama antibody biotech in Belgium, has now proven to be on solid ground regarding their orphan drug for a rare blood disease. Now the New England Journal of Medicine has published their phase II results.
 Ablynx often makes our biotech headlines, be it with the curious science of their nanobody platform or with their partnerships with big pharma companies.
Ablynx often makes our biotech headlines, be it with the curious science of their nanobody platform or with their partnerships with big pharma companies.
Despite being actively developing therapies in partnerships with big pharma for different diseases (including multiple sclerosis with Genzyme, cancer with MSD, and with NovoNordisk in a yet undisclosed disease), their flagship is a drug for the rare acquired thrombotic thrombocytopenic purpura (aTTP).
This blood disorder is an autoimmune disease which afflicts only about 5 people in a million per year, but can lead to severe complications (like brain damage and kidney failure). It is currently treated with a daily plasma exchange (TPE) – a sort of blood “cleaning”, that requires people to stay in the hospital for long periods of time, and can have complications of its own.
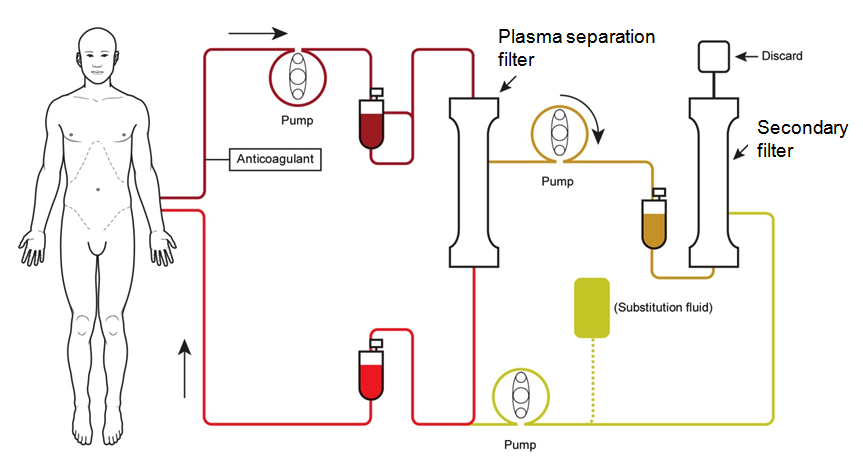
Ablynx is developing this drug, caplacizumab, alone, and had already disclosed positive topline results from their Phase II clinical trial (named ‘Titan‘) in 2014. Now they have published their data and results in one of the most prestigious medical journals, the New England Journal of Medicine.
With an impact factor of 55.873 (compared to The Lancet which is scored 45.17, or the BMJ at 14.5), the NEJM is the highest ranking peer-reviewed publication in general medicine. So the fact that Ablynx managed to publish in this particular journal should speak for the solidity of their results.
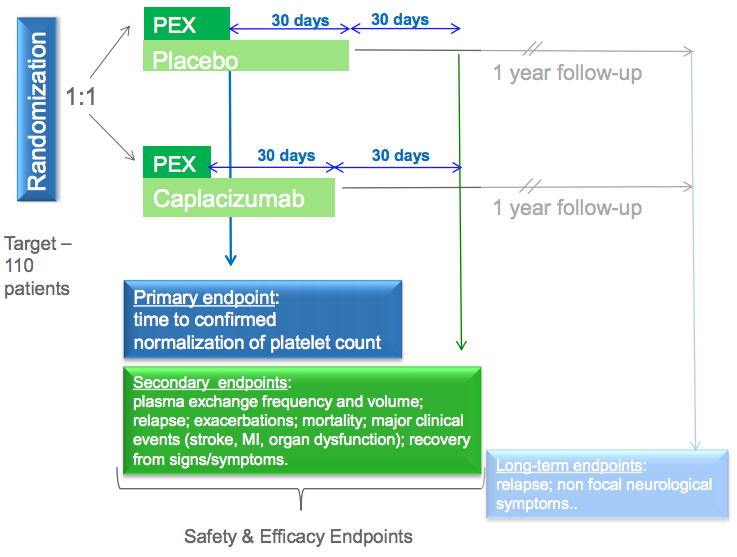
Along with the other 30 or so nanobodies they are investigating, Ablynx is pushing the potential commercialization of caplacizumab with a phase III clinical trial (‘Hercules‘) and by filing for conditional approval of this drug in Europe.
Although facing competition from other approaches like that of Delenex (Switzerland) and Argenx, which also uses the small antibodies of camiladae, Ablynx is one of the leaders in small antibody therapies. On the other hand, Argenx (also based in Belgium) is working with LEO Pharma with their antibodies in an autoimmune research partnership.
This publication is a great recognition of Ablynx’s work, and we are definitely looking forward to the results of Hercules…






