Newsletter Signup - Under Article / In Page
"*" indicates required fields
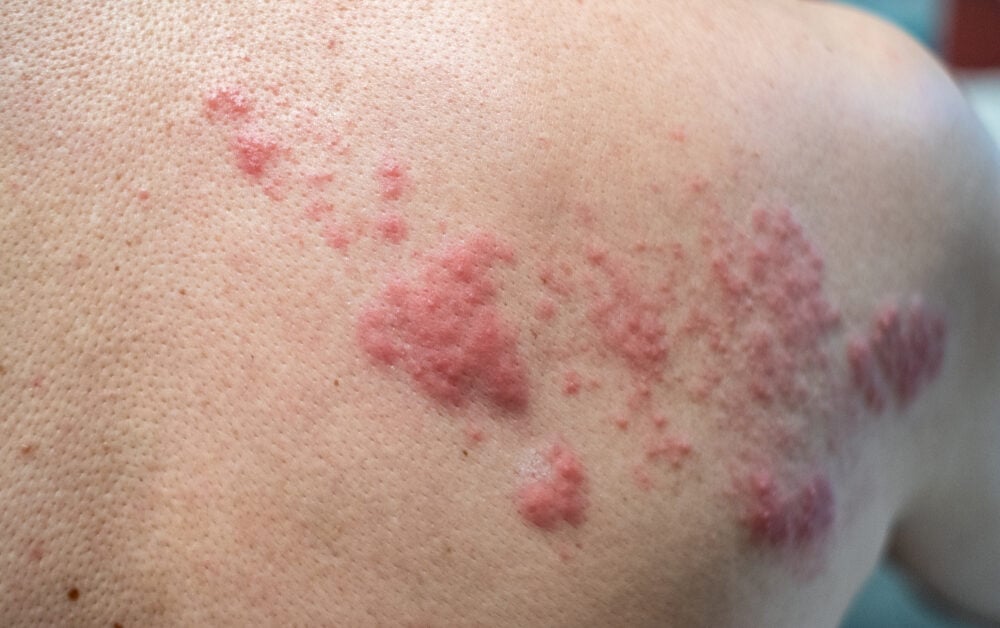
Jiangsu Recbio Technology Co., Ltd. has received notice of acceptance from China’s National Medical Products Administration (NMPA) to start a clinical trial for its self-developed novel adjuvanted recombinant shingles vaccine, REC610.
Within 60 days from the date of acceptance, the company may carry out clinical trials in accordance with the submitted plan if no negative or doubtful comments are received from the Center for Drug Evaluation of NMPA.
The company is proposing a randomized, double-blind, Shingrix parallel controlled phase I clinical trial in 180 healthy adult subjects aged 40 and above in China to evaluate the safety and tolerability of REC610, and have a preliminary assessment of its immunogenicity.
Shingles is a common viral infectious disease that seriously affects the quality of life of patients, especially elderly patients. It is estimated that more than 1.5 million new cases of shingles occur each year in people aged 50 and over in China. In recent years, the age of those contracting shingles has gradually lowered.
REC610 is equipped with a novel adjuvant BFA01 independently developed by the company, which can promote the production of high levels of VZV glycoprotein E (gE)-specific CD4+ T cells and antibody. REC610 is intended to prevent shingles in adults aged 40 and above. Preclinical studies have shown REC610 has favorable immunogenicity and can induce high levels of gE antigen-specific CD4+T cell responses and IgG antibodies, and its immune response is not inferior to the controlled vaccine Shingrix.
Previously, the company commenced the first-in-human GSK Shingrix active controlled clinical trial of REC610 in the Philippines in February 2023. All subjects have completed 30 days of follow-up studies after two doses of the vaccination, with favorable safety and tolerability profile.
About Recbio
Recbio has developed high-value vaccine portfolios consisting of differentiated vaccines, covering cervical cancer, shingles, COVID-19, TB and other high-burden diseases. Its core product, REC603, a recombinant 9-valent HPV vaccine in phase III clinical trial, shows promise of becoming the first marketed domestic 9-valent HPV vaccine.
ReCOV, a recombinant COVID-19 vaccine with commercialization in the works, is a next-generation COVID-19 vaccine.