CRISPR has received a regulatory green light in what could be the first clinical trial with the “scientific discovery of the century”. It’ll be used to improve cancer cell therapies.
![]() CRISPR has already revolutionized biotech, quickly overtaking the field of gene editing. It also has immense potential in the therapeutic field, so it’s not surprising there’s a lot of interest, not to mention its famous patent war.
CRISPR has already revolutionized biotech, quickly overtaking the field of gene editing. It also has immense potential in the therapeutic field, so it’s not surprising there’s a lot of interest, not to mention its famous patent war.
What may be surprising is the amount of money involved, with $120M mega-rounds by Editas Medicine (US), plus $70M for Intellia (US) and €79.5M for CRISPR Therapeutics (Switzerland). That’s a lot of money for something without any clinical data! ( a topic brought up during the CRISPR fireside chat at Refresh…)
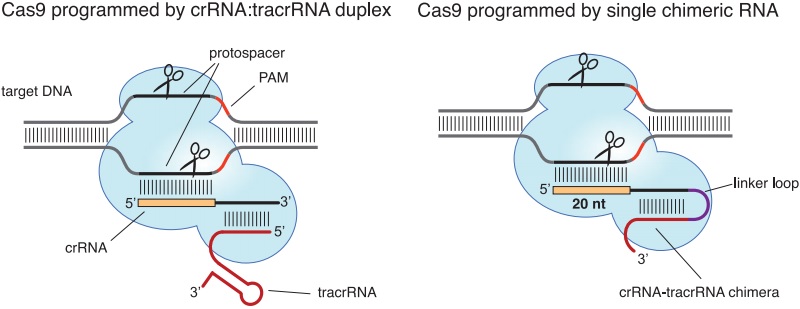
This clinical data is now one step closer to reality as the US National Institutes of Health (NIH) approved a study proposal to use CRISPR/Cas9 technology to improve cancer cell therapies. While it still needs FDA approval, this is a big step forward.
The trial will be backed by the Parker Foundation, founded by the tech billionaire Sean Parker (Napster co-founder and former Facebook president) – another example of Tech investors eyeing biotech.
As for the science, the University of Pennsylvania will edit the T-cells of 18 patients with melanoma, sarcoma, or myeloma. The university is also involved in the most advanced CAR-T trial, in collaboration with Novartis.
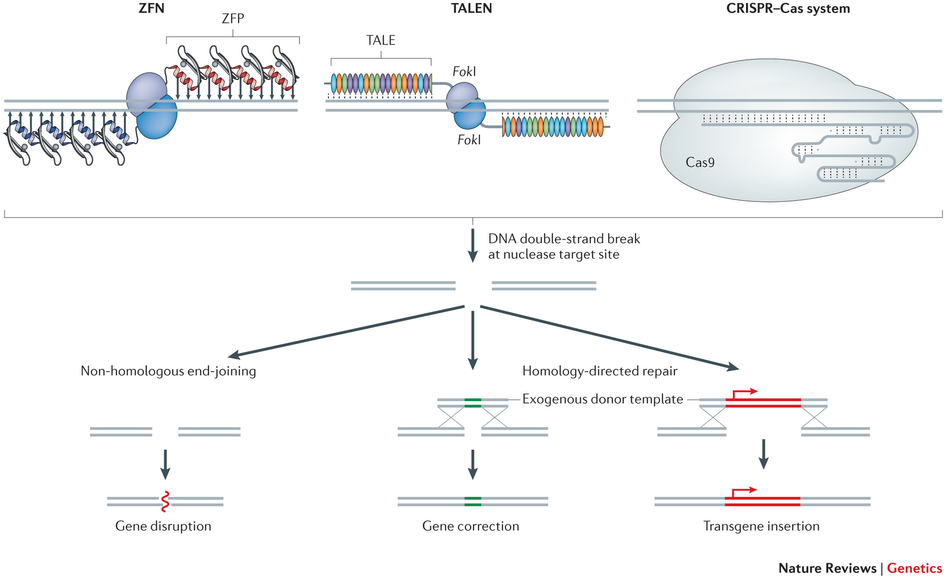
CRISPR-Cas9 will be used to insert a gene to better detect cancer cells and will suppress a gene expressing biomarkers on T-cells that are blocked by cancer cells.
This first trial will focus on safety. It’s not the first time that gene editing has been used in trials of cell therapies (with zinc-finger nucleases and TALENS), but CRISPR has some special safety concerns of its own – such as the possibility of off-target edition.
With this approval, the research team expects the trial could begin as soon as the end of 2016. Undoubtedly something to keep an eye on…
Feature Image Credit: Pixabay
Figure 1 Credit: Jinek et al. (2012) A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science (doi: 10.1126/science.1225829)
Figure 2 Credit: Yin et al. (2014) Non-viral vectors for gene-based therapy. Nature Review Genetics (doi: 10.1038/nrg3763)





