Genfit is extending the applications of its star candidate, Elafibranor, to a second liver disease – Primary Biliary Cholangitis (PBC), a rare condition with high unmet medical needs.
![]() Genfit from Lille (France) has been in the spotlight because of its phase III candidate for NASH – something we discussed with its CEO, Jean-François Mouney.
Genfit from Lille (France) has been in the spotlight because of its phase III candidate for NASH – something we discussed with its CEO, Jean-François Mouney.
Apparently, all the hype is not making Genfit lazy. It has just announced that it will diversify the diseases targeted by Elafibranor with a new trial in primary biliary cholangitis (PBC).
PBC is a chronic disease that causes the bile ducts to become inflamed, gradually destroying them. Bile ducts are a sort of internal plumbing to carry bile (which is secreted in the liver) to the intestine, where it is essential for food digestion.
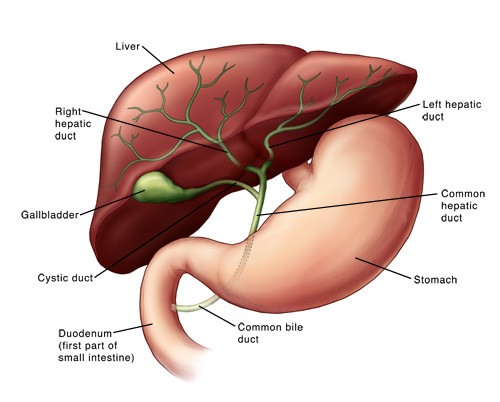
The current treatment for PBC is ursodeoxycholic acid (UDCA), which replaces bile acids. But around 70% of the patients don’t tolerate or don’t respond sufficiently to this therapy – which makes PBC an area of unmet medical need.
The choice of this disease was based on the known mechanistic effect of PPAR alpha (the pathway where Elifibranor acts) on bile acid metabolism, as well as positive effects seen on biomarkers (an area where Genfit is also quite active) during the NASH trials.
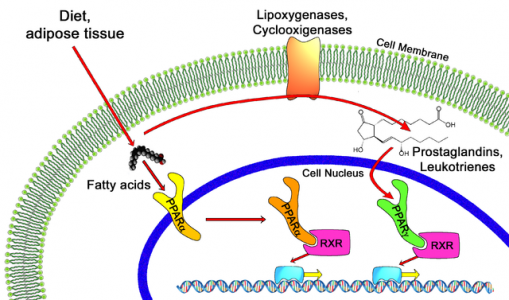
The clinical development of PBC will be built upon the previous safety trials of Elafibranor. This will allow Genfit to jump right into phase II, expected to start before the end of 2016.
Genfit made this announcement at its R&D Event in New York City. It also released other news (if not quite with the same impact), including the launch of new trials in NASH for pediatric and cirrhosis subpopulations.
Investors received this news positively – the stock is increasing by 5.8% while I’m writing.
Most known for its success in the NASH field, Genfit looks committed to develop a more robust pipeline. This new trial in a second liver condition is really exciting news.