Microbiomics is a hot area for biotech. Now US Seres Therapeutics and Nestlé Health Sciences have signed a massive drug development deal for bowel diseases and C.difficile infections.
 By correcting the fundamental microbiome dysbiosis that is the root cause of many diseases, Seres Theraputics in Cambridge (US) is creating a profoundly new and important way of treating many chronic gastrointestinal diseases.
By correcting the fundamental microbiome dysbiosis that is the root cause of many diseases, Seres Theraputics in Cambridge (US) is creating a profoundly new and important way of treating many chronic gastrointestinal diseases.
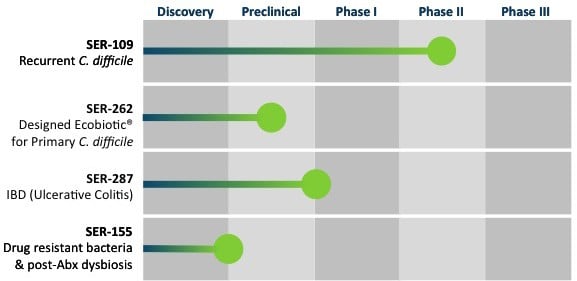
In addition to connections with Moderna and Flagship Ventures, Seres has now also entered into an agreement with Nestlé Health Science in Epalinges (Switzerland) for the development and commercialization for its product candidates (SER-109 and SER-262) for Clostridium difficile infections (CDI).
Candidates SER-287 and SER-301 against inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, will also be included in the partnership program.
Nestlé Health Science agreed to provide Seres with an upfront payment of €110.5M ($120M) in cash and a series of development and sales milestones and tiered royalties.
Seres then expects to receive a total of €27.6M ($30M) in milestone payments in 2016 associated with the planned initiation of a Phase Ib study for SER-262 in primary CDI, and the anticipated start of the Phase III trial for SER-109 in recurrent CDI.
Seres’ most advanced program, SER-109, has successfully completed a Phase Ib/II study demonstrating a clinical benefit in patients with recurring Clostridium difficile infections and is currently being evaluated in a Phase II study for these. The FDA has even granted it Orphan Drug, as well as Breakthrough Therapy, designations, considering the impact CDI’s can have on public health.
The full potential value of the up-front payment, milestones and royalties payable by Nestlé Health Science is over €1.7Bn ($1.9Bn), assuming all products receive regulatory approval and are successfully commercialized.
We visited Seres Therapeutics (originally Seres Health)in Boston back in 2014, just a few months before their IPO and Nestlé’s investment of $65M into Seres was announced. As their CEO explained:
“[We have] identified the microbiome as an area of strategic importance for our emerging novel therapeutics practice, and we expect that investing in an industry leader like Seres Health will help us reach our ambitions of addressing health conditions in the area of Gastrointestinal and Metabolic Health.”
And this new, ambitious partnership is just the latest example of how the Microbiomics field is strengthening TransAtlantic ties for therapeutic developments in chronic disease.