Newsletter Signup - Under Article / In Page
"*" indicates required fields
The French company Sensorion has been forced to delay a phase II trial in people with hearing loss due to slow patient recruitment amid the current coronavirus disease (Covid-19) pandemic.
The clinical trial is designed to test Sensorion’s lead candidate drug in people with sudden sensorineural hearing loss, a rare disorder where the patient can go deaf within several days. The trial results were to be released late 2020, but the company now expects to release them in mid-2021.
Nawal Ouzren, CEO of Sensorion, stated that slow patient recruitment caused the delay, as well as hospital resources being reprioritized to handle the Covid-19 outbreak. The global outbreak — labeled a pandemic by the World Health Organization last week — threatens to overwhelm healthcare systems around the world without an effective vaccine or drug approved for the disease.
The company stated that it will keep monitoring the Covid-19 situation, which is changing very quickly. “We are doing our utmost to ensure we can provide the clinical data set as quickly as possible,” stated Géraldine Honnet, Sensorion’s CMO.
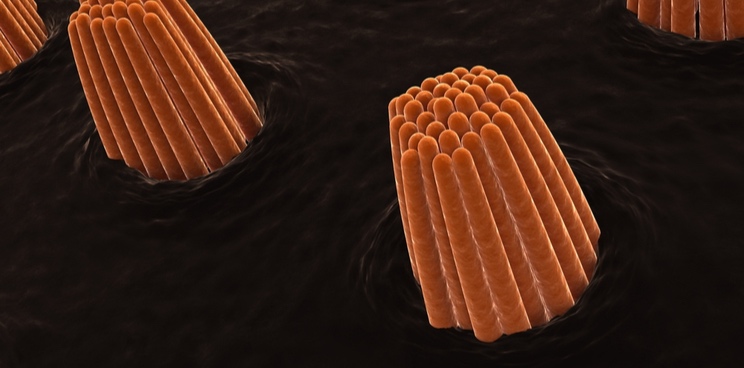
Sudden sensorineural hearing loss occurs when the sensory hair cells in the inner ear are destroyed, most often for an unknown reason. Sensorion’s drug is designed to treat the condition by protecting hair cells from damage.
Sensorion raised €20M in a bonds issue last year to fund the phase II development of treatments for sudden deafness and acute unilateral vestibulopathy, a rare disorder causing vertigo. The company was confident that the funding would sustain the programs until late 2020.
However, this latest delay, along with the phase II failure and discontinuation of its treatment for vertigo in December 2019, have proved big setbacks for the French company. Its Euronext Paris stock price — which had almost recovered from a 50% drop caused by its phase II failure — has dropped again by 30% since the market opened today.
Covid-19 is a fairly new experience for modern healthcare, as a pandemic on this scale has not been seen in recent history. This is causing clinical trial delays across the board. It remains to be seen how companies will be able to navigate this difficult situation and minimize the impact on their pipelines.
Images from Shutterstock