Chikungunya fever could be the next big global outbreak. Reassuringly, Themis’ vaccine has so far performed well in a Phase II study.
Vienna-based biotech, Themis, develops urgently needed vaccines against diseases, which have the potential to be the next global outbreak. The company has announced encouraging preliminary Phase II results for its vaccine against chikungunya fever. Next, Themis will hope that the study’s final results, which are expected in 2018 and 2019, continue to suggest that its vaccine could provide effective protection from the tropical disease.
Chikungunya fever is a viral infection transmitted by Aedes mosquitoes. It originates in Africa but increased global travel and rising temperatures have caused the mosquitoes carrying the virus to spread. Over the last four years, there have been over 1.7 million cases across 45 countries. The virus causes sudden fever and severe joint pain, which can become chronic.
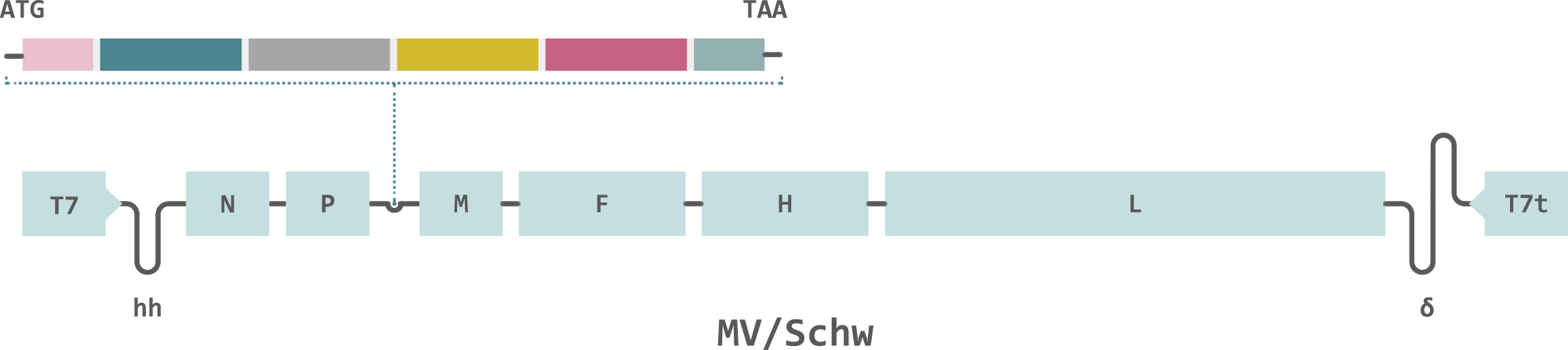
Themis’ candidate is a modified measles vaccine that expresses antigens from the Chikungunya virus. The vaccine delivers the viral antigens directly to macrophages and dendritic cells, which triggers a strong immune response. The vector is able to replicate so continuously expresses antigens after immunization, resulting in long-term immunity.
Unfortunately, tropical diseases are often forgotten about until a major outbreak takes place and lives are lost. Thankfully, biotech has stepped in and is taking a number of different approaches to tackle these deadly diseases. Swiss biotech, Humabs, isolated an antibody against Middle East Respiratory Syndrome and has programs against Zika and Dengue viruses at the preclinical stage. Oxford University spin-out, Oxitec, has opened a factory in Brazil to continue producing a genetically engineered Aedes aegypti mosquito, which can limit the population size.
Earlier this year, Themis was boosted by a grant of £3M (€3.3M) from Innovate UK to push the vaccine towards Phase III, and it can be pleased with the progress made so far. The money from Innovate UK will help Themis to prepare for Phase III, by giving it the funds to conduct a small phase I study in the UK to validate the vaccine’s correlate of protection and identify a protective antibody threshold.
It is refreshing to see a company learning from our mistakes in the fight against previous outbreaks like Ebola and Zika. There has already been one major outbreak of Chikungunya fever back in 2015, so let’s hope that Themis has its vaccine ready if or when there is another.
Images – khlungcenter / shutterstock.com; Themis





