Newsletter Signup - Under Article / In Page
"*" indicates required fields
Immunotherapy – A big word with much meaning. There’s no use in talking about their history. The fact that immunotherapies seem to be the next big thing in biotech is reason enough to discuss them.
For this purpose, we have caught up with Etienne Drouet, Vice President of Strategic Development at SynteractHCR, an international CRO. Together, we have discussed the ‘big five’ of immunotherapies.
Starting from their various types and applications, we then talked about the key facts biopharmaceutical companies need to know when planning immunotherapy trials and the important role that CROs play. Lastly, Etienne gave us an insight into the future of immunotherapies.
The Application of Immunotherapies in Other Disease Areas
Although originally developed as a treatment against cancer, immunotherapies are slowly being used in other disease areas as well. Researchers are now investigating a wide variety of indications, including genetic disorders, inflammation, diabetes, cardiovascular diseases and regenerative medicine.
“The potential of immunotherapies is huge. I would say that immunotherapies are the most important change in oncology treatment in the past 10 years, and now they are expanding into other disease areas,” says Etienne. “Immunotherapy is the new star in the life sciences. Compared to chemotherapy they have less adverse events, and also show positive long-term effects in overall survival rates.”
The Different Types of Immunotherapies
As they play a major role in the body’s immune system, monoclonal antibodies (mAbs) are the most traditional form of immunotherapy. Today, however, many other techniques and agents have joined the list, including checkpoint inhibitors, gene or cell therapy and vaccines.
In the case of cancer, many of these treatments have changed the standards compared to traditional therapies, such as chemo– or radiotherapy to fight the disease. As immunotherapies can be personalized, standardized or generic, their combination with other therapies can differ widely from one indication to another and from one patient to another.
“The variety in the panel of immunotherapies is rapidly increasing. Vaccines are not recent, but with improved technologies a new and more immunogenic generation of vaccines with specific cancer antigens from individual patients is being developed. Personalized immunotherapies are definitely a growing field,” Etienne explains.
Monoclonal Antibodies – what can they do?
The application of mAbs in anti-cancer treatment is very versatile. Naked mAbs are usually designed to bind to a specific antigen on the surface of tumour cells, to the effect of preventing the cell’s reproduction or acting as a marker for the immune system to attack.
On the other hand, conjugated mAbs, known as ADCs (antibody-drug conjugates), act as toxin carriers. They can release the cytotoxic agents directly inside the cancer cell or on its surface, avoiding negative effects on the body’s healthy cells.
Lastly, bispecific mAbs can bind to two different target proteins at the same time. For instance, they can mediate an immune reaction by binding to the tumor cell and a T-cell, triggering an attack on the cancer cells.
Checkpoint Inhibitors – supporting drugs are the next big thing!
Checkpoints are small molecules located on specific immune cells, which trigger an immune response when they are activated or deactivated. But there is a problem:
“Checkpoint inhibitors were developed 10 years ago. Although they are really effective, there is still a large part of the patient population that isn’t responding to this drug,” says Etienne.
“If you look at the response rate for melanoma or non-small cell lung cancer, it’s at 40-60% at its best. So the next development efforts are being done on drugs that boost the effectivity of checkpoint inhibitors, aiming to increase the response rate in a large group of the population.”
Scancell and Idera Pharmaceuticals, for instance, are companies currently working on developing drugs that support checkpoint inhibitors and increasing their impact on cancer cells. Both are focusing on the checkpoint protein PD-1, which can be found on the surface of T-cells.
PD-1 is responsible for keeping T-cells switched off, as they would otherwise attack the body’s healthy cells. Drugs, such as those developed by Scancell and Idera, can block PD-1 and boost immune responses.
Gene and Cell Therapy – personalized treatments are increasing
In August 2017, Novartis was the first company to get the first ever gene therapy – known under the general term CAR-T therapy – against acute lymphoblastic leukemia (ALL) approved by the FDA.
Now, biotech companies worldwide have entered a neck-and-neck race to develop a cheaper and more effective version of Novartis’ Kymriah. The price of close to half a million US dollars is, at least partially, explained by the complicated manufacturing process of the CAR-T therapy.
Etienne explains: “The treatment is personalized, meaning that the patient’s own T-cells are extracted from the body, genetically manipulated to recognize a specific antigen on malignant B-cells, and then transferred back into the patient, where they can fight leukemia. Although the manufacturing process is complicated, the treatment only needs to be done once, and this massively decreases the logistical problems that I will talk about later.”
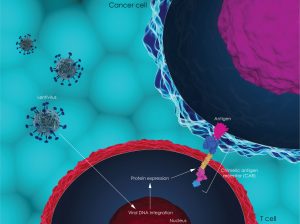
Until now, the CAR-T therapy has a high efficiency, but side effects remain a serious concern. Adverse events mainly take on the form of immuno-side effects, such as autoimmune diseases or life-threatening cytokine storms. “A lot more work has to be done in this area, especially patient follow-ups after a longer period of time,” Etienne emphasizes.
Vaccines can be based on many different ‘ingredients’
Unlike preventative vaccines, anti-cancer vaccines are administered to support the patient’s immune response. These vaccines can be made up of cells from the immune system, such as dendritic cells, the RNA of tumor antigens, DNA, or recombinant viruses. They can be autologous – based on the patient’s own immune cells, or allogeneic – based on a tumor antigen largely present on a defined tumor entity.
“The manufacturing step for RNA or DNA-based vaccines is much simpler than the creation of cell-based vaccines,” Etienne explains. “In the US, Dendreon’s Provenge cell-based vaccine against prostate cancer was the only cell-based anti-cancer vaccine to be approved by the FDA before the CAR-t therapy.

“It is also personalized, so a patient’s own tumor cells are used to activate his or her immune system. For each patient, a type of immune cell called dendritic cells, are extracted and basically trained outside of the body, inside the lab to recognize and attack the patient’s own prostate cancer cells. Dendritic cells are naturally involved in the activation of the immune system.”
Many more companies are focusing on developing vaccines with oncolytic viruses, the most famous of which is Amgen’s Imlygic or T-VEC. Approved in late 2015, T-VEC is used for patients with melanoma.

The idea behind this therapy is based on the hypothesis that the virus infects and kills cancer cells, while leaving the body’s healthy cells intact and boosting the patient’s immune system at the same time. “The strong immune response can be helpful, but if a patient is immuno-depressed, the vaccine might not be as effective,” concedes Etienne.
What Biopharmaceutical Companies Need to Know When Planning Immunotherapy Drug Trials
There are several factors that should be taken into account during the planning phase of an immunotherapy drug trial. Apart from the more obvious regulatory and safety procedures, biopharma companies should also consider logistics and evaluation processes.

“It depends a lot on the kind of drug you are developing,” answers Etienne. “Obviously, the competition has to be kept in mind: What kind of drugs are being developed in this area? What will be on the market? How is the standard changing and what is the future outlook on this specific therapeutic area? All important questions to be considered.”
Logistics – a blessing or a curse?
“For personalized or cell-based treatments, logistical and manufacturing processes have to be taken into consideration. Does it make sense to develop the drug, or is it too complicated? Companies should consider factors, such as shelf-life or manufacturing time. If a drug takes more than 10-12 weeks to be produced, there’s a high risk that the patient progresses during that time. For personalized drugs that require a repeated treatment on multiple occasions, the logistics become very complex to manage.”

Etienne emphasizes the importance of a stringent development and manufacturing plan, as well as the importance of tightly regulated shipping and security procedures. As drugs can be extremely expensive – remember Novartis’ Kymriah for $475,000 – there is a high danger of theft or misuse. Consequently, drugs have to be efficiently tracked and protected.
Patient Safety, Screening and Regulation – don’t forget these details and get help!
Not only the safety of the medication, but also the safety of the patient is a key factor in the planning of immunotherapy drug trials – or any other drug trial for that matter. To be eligible for a trial, patients have to be screened in a lengthy process that analyzes potential risks or failures.
Criteria for the evaluation of immunotherapy effectiveness are very different to those of other therapies. This can be traced back to the fact that a patient’s own immune system is used to fight cancer. Often, a pseudo progression or a delayed response to the treatment can take place, making the evaluation harder and the modification of evaluation protocols necessary.

As a consequence, the whole regulatory process for immunotherapy drugs is lengthy and complicated. In the case of genetically engineered cells, stricter regulations require the assistance of regulatory specialists who are experienced in the organization and proceedings of immunotherapy trials and the communication with necessary authorities.
The Important Role of CROs in Planning Immunotherapy Drug Trials
“We provide support in planning, monitoring and the involvement of different stakeholders, whether regulatory or medical. Using a CRO for assistance is important, as immunotherapy drugs or gene therapies are very complex to handle in a clinical trial,” explains Etienne.
“When it comes to personalized medicine, SynteractHCR can assist companies starting with the initial steps before drug production, through the whole cycle of production and the coordination of all partners. We have experience in the majority of immuno-oncology treatments, such as checkpoint inhibitors, bispecific antibodies, RNA and DNA based vaccines, personalized treatment with dendritic cell vaccines or adoptive T-cell therapies.

“In other words, a CRO plays an important role in the management of a trial: assessing regulatory processes, confirming measurements, or determining additional clinical evaluation artefacts that may arise exclusively in the course of the immunotherapy.”
New trends in Phase I and II trials
Etienne explains that there is a new trend in the organization of Phase I and II trials: “There is a trend to do more with these first two phases, to try more combinations, different settings and so on, to narrow down the patient population in Phases I and II, and to make sure that strong efficacy signals are identified before going to Phase III.”
Historically speaking, he says, chemotherapy trials were designed to reach Phase III as quickly as possible, so researchers could contain large statistical samples with small differences. Nowadays, however, the trend is to try and identify the pool of patients in which the drug is most effective at the earliest stage possible.

“That way you don’t need as many patients and you get a more dramatic difference between your control and patient groups. It also means that small companies can afford this adaptive design, in which multiple scenarios are tested. But, of course, this requires a lot of planning and managerial, medical and regulatory support, which is where immunotherapy-experienced CROs come in handy.”
The Future of Immunotherapies
Resulting from the wider scope of application areas is the increased number of companies investing in therapies. In the near future, the size of the immunotherapy market is expected to grow exponentially, and the cancer immunotherapy market alone is expected to surpass $120bn by 2021, compared to a mere $62bn in 2016.
Drugs that support checkpoint inhibitors seem to be the rising stars of immunotherapy. As many patients are not responding to checkpoint inhibitors alone, these drugs address the unmet needs of this patient group. The main hope of these drugs is to increase the effect of checkpoint inhibitors and convert a partial immune response to a complete response.
“In future, new treatments such as CAR-T therapy will be another step forward in the fight against cancer. CAR-T is the first step, and then there are other technologies that might be integrated in the anti-cancer movement, like CRISPR Cas9,” concludes Etienne. He looks forward to positive advances towards much-needed treatment options.
Want to learn more about immunotherapies? Check out SynteractHCR’s website and learn more about the important role of CROs in planning immunotherapy drug trials!
Images via Kateryna Kon, media point inc, Alfa Photo, Skintone studio, SFIO CRACHO, Zolnierek, Numstocker, Natali_Mis, Verras, fusebulb, bluebay, Juan Gaertner, Christoph Burgstedt, vitstudio/Shutterstock.com






